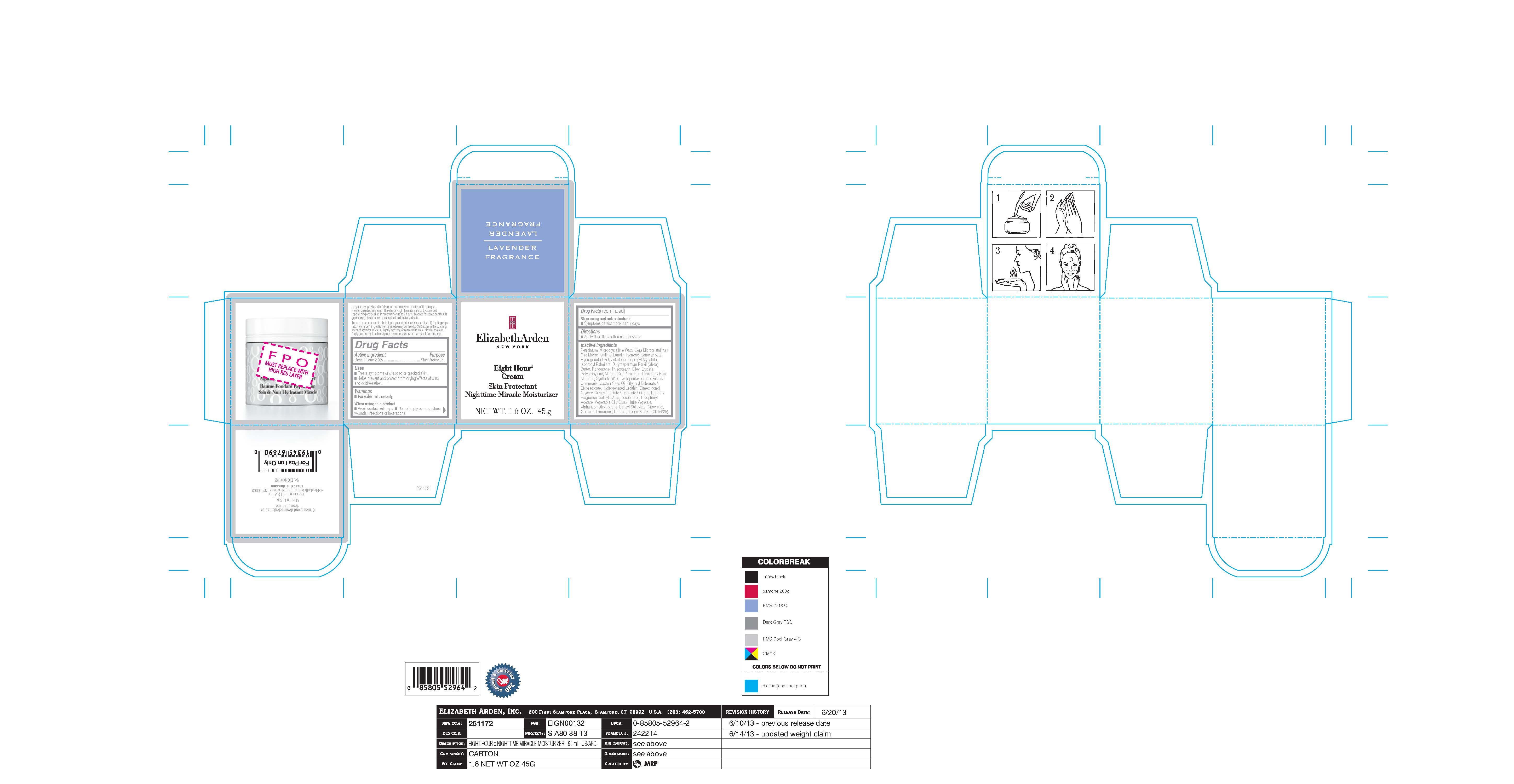
Label: EIGHT HOUR NIGHT- dimethicone emulsion
- NDC Code(s): 10967-675-16
- Packager: REVLON
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
-
Inactive Ingredients
PETROLATUM, MICROCRYSTALLINE WAX/CERA MICROCRISTALLINA/CIRE MICROCRISTALLINE, LANOLIN, ISONONYL ISONONANOATE, HYDROGENATED POLYISOBUTENE, ISOPROPYL MYRISTATE, ISOPROPYL PALMITATE, BUTYROSPERMUM PARKII (SHEA) BUTTER, POLYBUTENE, TRIISOSTEARIN, OLEYL ERUCATE, POLYPROPYLENE, MINERAL OIL/PARAFFINUM LIQUIDUM/HUILE MINERALE, SYNTHETIC WAX, CYCLOPENTASILOXANE, RICINUS COMMUNIS (CASTOR) SEED OIL, GLYCERYL BEHENATE/EICOSADIOATE, HYDROGENATED LECITHIN, DIMETHICONOL, GLYCERYL CITRATE/LACTATE/LINOLEATE/OLEATE, PARFUM/FRAGRANCE, SALICYLIC ACID, TOCOPHEROL, TOCOPHERYL ACETATE, VEGETABLE OIL/OLUS/HUILE VEGETALE, ALPHA-ISOMETHYL IONONE, BENZYL SALICYLATE, CITRONELLOL, GERANIOL, LIMONENE, LINALOOL, YELLOW 6 LAKE (CI 15985).
- Uses and Directions
- Warnings
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- carton art
-
INGREDIENTS AND APPEARANCE
EIGHT HOUR NIGHT
dimethicone emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-675 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 2 mg in 1 g Inactive Ingredients Ingredient Name Strength ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) SALICYLIC ACID (UNII: O414PZ4LPZ) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LIMONENE, (+)- (UNII: GFD7C86Q1W) GLYCERYL BEHENATE/EICOSADIOATE (UNII: 73CJJ317SR) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) SYNTHETIC WAX (1200 MW) (UNII: Q3Z4BCH099) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) SHEA BUTTER (UNII: K49155WL9Y) OLEYL ERUCATE (UNII: 753W099NQ6) PETROLATUM (UNII: 4T6H12BN9U) MINERAL OIL (UNII: T5L8T28FGP) TOCOPHEROL (UNII: R0ZB2556P8) CORN OIL (UNII: 8470G57WFM) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) LANOLIN (UNII: 7EV65EAW6H) DIMETHICONOL (100000 CST) (UNII: OSA9UP217S) GERANIOL (UNII: L837108USY) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) POLYPROPYLENE (30000 MW) (UNII: T71QXI2O62) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-675-16 45 g in 1 JAR; Type 0: Not a Combination Product 10/20/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 10/20/2020 Labeler - REVLON (788820165) Registrant - REVLON (788820165) Establishment Name Address ID/FEI Business Operations Revlon 809725570 manufacture(10967-675)