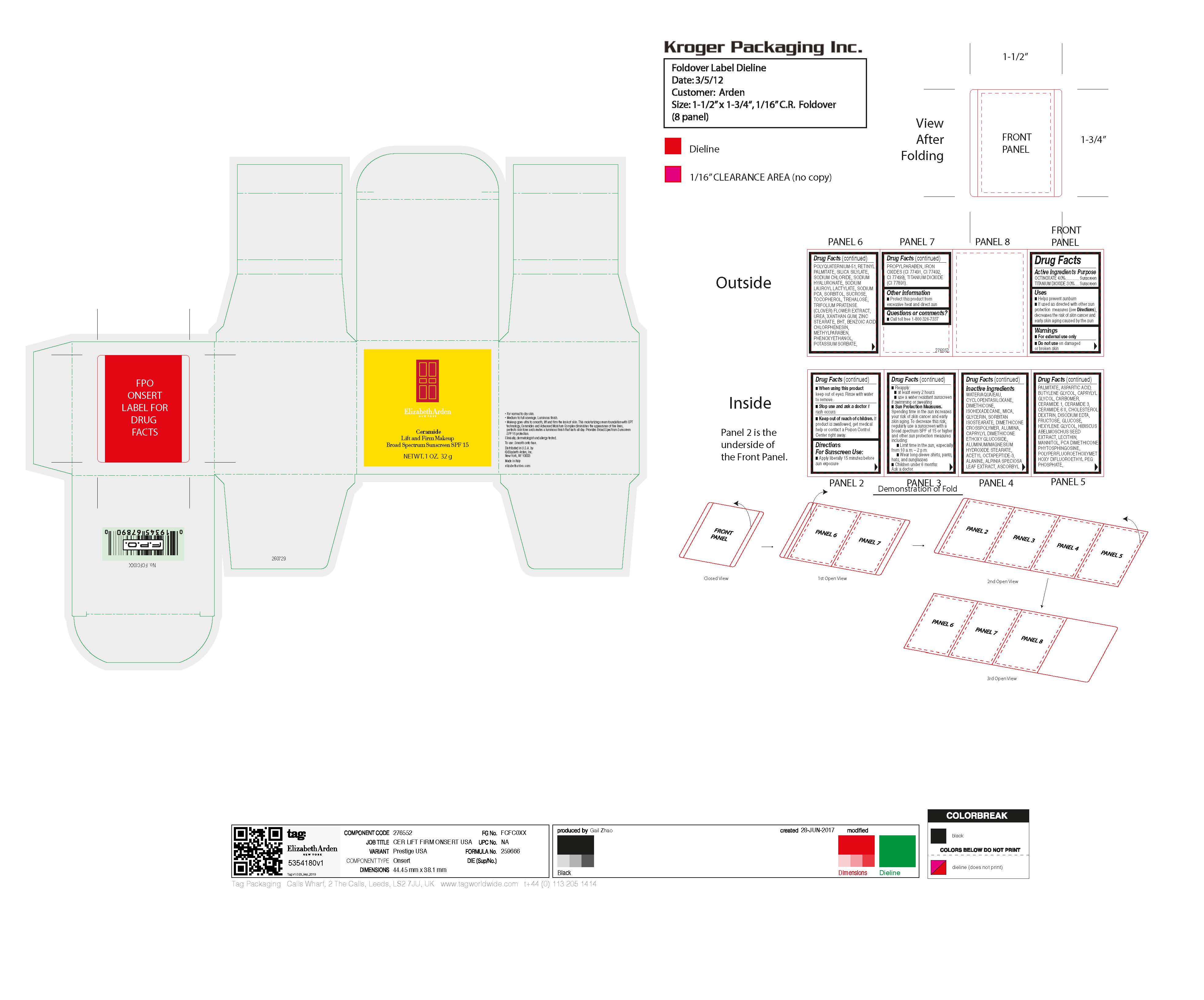
Label: CERAMIDE MAKEUP SPF 15- octinoxate, titanium dioxide cream
- NDC Code(s): 10967-668-01
- Packager: REVLON
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients Purpose
-
Inactive Ingredients
Inactive Ingredients
WATER/AQUA/EAU, CYCLOPENTASILOXANE, DIMETHICONE, ISOHEXADECANE, MICA, GLYCERIN, SORBITAN ISOSTEARATE, DIMETHICONE CROSSPOLYMER, ALUMINA, CAPRYLYL DIMETHICONE ETHOXY GLUCOSIDE, ALUMINUM/MAGNESIUM HYDROXIDE STEARATE, ACETYL OCTAPEPTIDE-3, ALANINE, ALPINIA SPECIOSA LEAF EXTRACT, ASCORBYL PALMITATE, ASPARTIC ACID, BUTYLENE GLYCOL, CAPRYLYL GLYCOL, CARBOMER, CERAMIDE 1, CERAMIDE 3, CERAMIDE 6 II, CHOLESTEROL, DEXTRIN, DISODIUM EDTA, FRUCTOSE, GLUCOSE, HEXYLENE GLYCOL, HIBISCUS ABELMOSCHUS SEED EXTRACT, LECITHIN, MANNITOL, PCA DIMETHICONE, PHYTOSPHINGOSINE, POLYPERFLUOROETHOXYMETHOXY DIFLUOROETHYL PEG PHOSPHATE, POLYQUATERNIUM-51, RETINYL PALMITATE, SILICA SILYLATE, SODIUM CHLORIDE, SODIUM HYALURONATE, SODIUM LAUROYL LACTYLATE, SODIUM PCA, SORBITOL, SUCROSE, TOCOPHEROL, TREHALOSE, TRIFOLIUM PRATENSE (CLOVER) FLOWER EXTRACT, UREA, XANTHAN GUM, ZINC STEARATE, BHT, BENZOIC ACID, CHLORPHENESIN, METHYLPARABEN, PHENOXYETHANOL, POTASSIUM SORBATE, PROPYLPARABEN, IRON OXIDES (CI 77491, CI 77492, CI 77499), TITANIUM DIOXIDE (CI 77891). - Uses
-
Warnings
Warnings
- For external use only
- Do not use on damaged or broken skin- When using this product keep out of eyes. Rinse with water to remove.
- Stop use and ask a doctor if rash occurs.
- Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
-
Directions For Sunscreen Use
Directions For Sunscreen Use:
- Apply liberally 15 minutes before sun exposure
- Reapply:- at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. – 2 p.m.
- Wear long-sleeve shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor.
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- carton art
-
INGREDIENTS AND APPEARANCE
CERAMIDE MAKEUP SPF 15
octinoxate, titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-668 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 3 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 4 mg in 1 g Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) TREHALOSE (UNII: B8WCK70T7I) TRIFOLIUM PRATENSE FLOWER (UNII: 4JS0838828) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) BENZOIC ACID (UNII: 8SKN0B0MIM) METHYLPARABEN (UNII: A2I8C7HI9T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) CAPRYLYL GLYCOL (UNII: 00YIU5438U) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) FRUCTOSE (UNII: 6YSS42VSEV) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ALANINE (UNII: OF5P57N2ZX) ALPINIA ZERUMBET LEAF (UNII: MS8P33AMKX) ABELMOSCHUS MOSCHATUS SEED (UNII: UN2QZ55I88) CHOLESTEROL (UNII: 97C5T2UQ7J) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) ACETYL OCTAPEPTIDE-3 (UNII: 8K14HJF88S) MANNITOL (UNII: 3OWL53L36A) ASPARTIC ACID (UNII: 30KYC7MIAI) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CERAMIDE 1 (UNII: 5THT33P7X7) PROPYLPARABEN (UNII: Z8IX2SC1OH) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) ISOHEXADECANE (UNII: 918X1OUF1E) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) ASCORBYL PALMITATE (UNII: QN83US2B0N) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) CERAMIDE 3 (UNII: 4370DF050B) CERAMIDE 6 II (UNII: F1X8L2B00J) PHENOXYETHANOL (UNII: HIE492ZZ3T) UREA (UNII: 8W8T17847W) XANTHAN GUM (UNII: TTV12P4NEE) CHLORPHENESIN (UNII: I670DAL4SZ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERIN (UNII: PDC6A3C0OX) MICA (UNII: V8A1AW0880) ALUMINUM OXIDE (UNII: LMI26O6933) DIMETHICONE (UNII: 92RU3N3Y1O) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SODIUM CHLORIDE (UNII: 451W47IQ8X) SORBITOL (UNII: 506T60A25R) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-668-01 32 g in 1 JAR; Type 0: Not a Combination Product 11/11/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/11/2015 Labeler - REVLON (788820165) Registrant - REVLON (788820165) Establishment Name Address ID/FEI Business Operations Revlon 809725570 manufacture(10967-668)