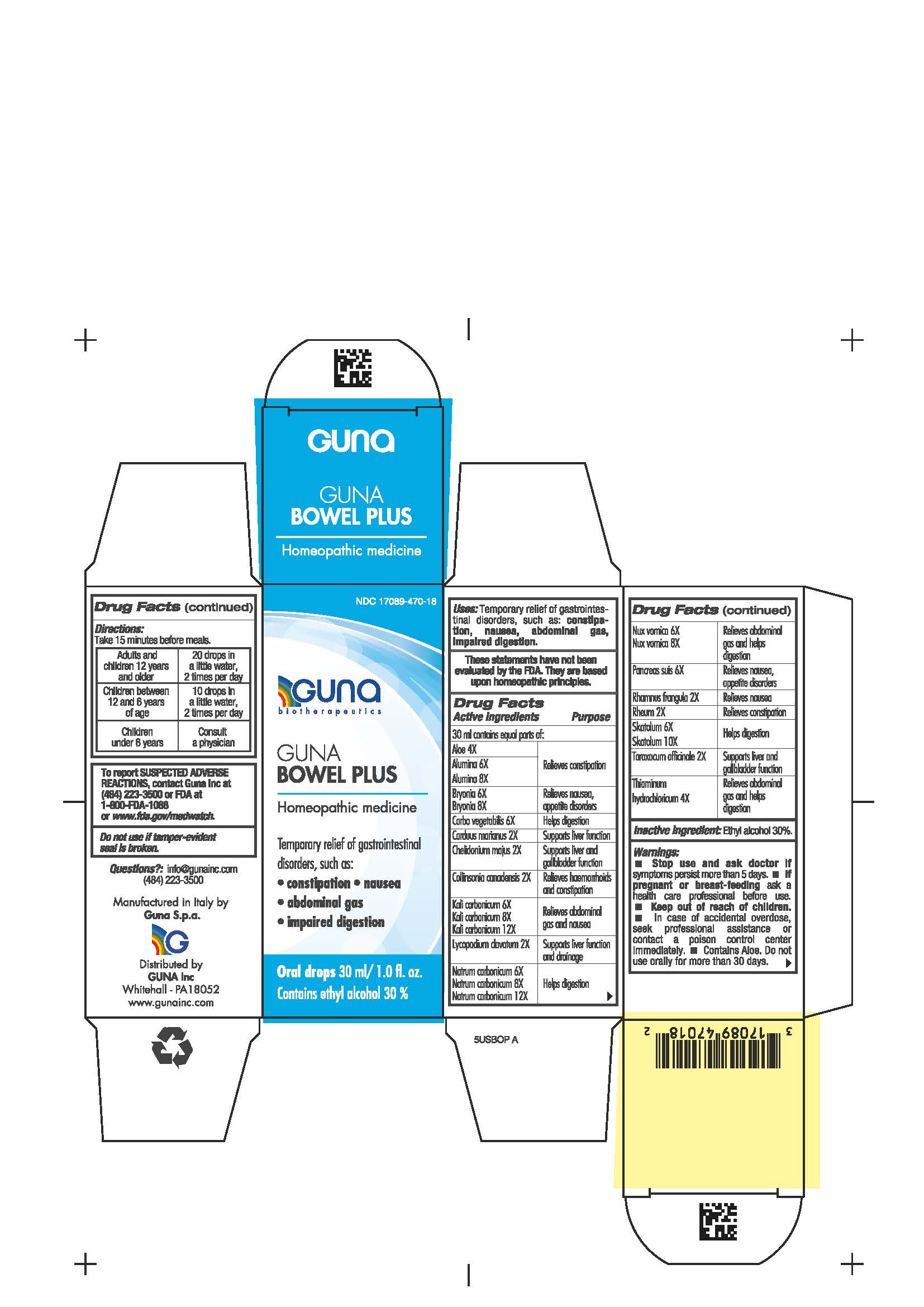
Label: GUNA BOWEL PLUS- activated charcoal - aloe - aluminum oxide - bryonia alba root - chelidonium majus - collinsonia - frangula alnus bark - lycopodium clavatum spore - potassium carbonate- rhubarb - strychnos nux vomica seed - silybum marianum seed - skatole - sodium carbonate - sus scrofa pancreas - taraxacum officinale - thiamine - solution/ drops
- NDC Code(s): 17089-470-18
- Packager: Guna spa
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 17, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- QUESTIONS
- DIRECTIONS
- WARNINGS
- PREGNANCY
-
WARNINGS
- Stop use and ask doctor if symptoms persist more than 5 days.
- If pregnant or breast-feeding ask a health care professional before use.
- Keep out of reach of children. In case of accidental overdose, seek professional assitance ot or contact a Poison Control Center immediately
- Contains Aloe: do not use orally for more than 30 days.
- Contains ethyl alcohol 30%
- USES
-
ACTIVE INGREDIENTS/PURPOSE
Aloe 4X Relieves constipation
Alumina 6X, 8X Relieves constipation
Bryonia 6X, 8X Relieves nausea, appetite disorders
Carbo vegetabilis 6X Helps digestion
Carduus marianus 2X Supports liver function
Chelidonium majus 2X Supports liver and gallbladder function
Collinsonia canadensis 2X Relieves hemorrhoids and constipation
Kali carbonicum 6X, 8X, 12X Relieves abdominal gas and nausea
Lycopodium clavatum 2X Supports liver function and drainage
Natrum carbonicum 6X, 8X, 12X Helps digestion
Nux vomica 6X, 8X Relieves abdominal gas and helps digestion
Pancreas suis 6X Relieves nausea, appetite disorders
Rhamnus frangula 2X Relieves nausea
Rheum 2X Relieves constipation
Skatolum 6X, 10X Helps digestion
Taraxacum officinale 2X Supports liver and gallbladder function
Thiaminum hydroc. 4X Relieves abdominal gas and helps digestion
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA BOWEL PLUS
activated charcoal - aloe - aluminum oxide - bryonia alba root - chelidonium majus - collinsonia - frangula alnus bark - lycopodium clavatum spore - potassium carbonate- rhubarb - strychnos nux vomica seed - silybum marianum seed - skatole - sodium carbonate - sus scrofa pancreas - taraxacum officinale - thiamine - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-470 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALOE (UNII: V5VD430YW9) (ALOE - UNII:V5VD430YW9) ALOE 4 [hp_X] in 30 mL ALUMINUM OXIDE (UNII: LMI26O6933) (ALUMINUM OXIDE - UNII:LMI26O6933) ALUMINUM OXIDE 6 [hp_X] in 30 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 6 [hp_X] in 30 mL SILYBUM MARIANUM SEED (UNII: U946SH95EE) (SILYBUM MARIANUM SEED - UNII:U946SH95EE) SILYBUM MARIANUM SEED 2 [hp_X] in 30 mL CHELIDONIUM MAJUS (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS 2 [hp_X] in 30 mL ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 6 [hp_X] in 30 mL COLLINSONIA (UNII: J9BTD5377V) (COLLINSONIA - UNII:J9BTD5377V) COLLINSONIA 2 [hp_X] in 30 mL SUS SCROFA PANCREAS (UNII: 9Y3J3362RY) (SUS SCROFA PANCREAS - UNII:9Y3J3362RY) SUS SCROFA PANCREAS 6 [hp_X] in 30 mL POTASSIUM CARBONATE (UNII: BQN1B9B9HA) (CARBONATE ION - UNII:7UJQ5OPE7D) POTASSIUM CARBONATE 6 [hp_X] in 30 mL SODIUM CARBONATE (UNII: 45P3261C7T) (CARBONATE ION - UNII:7UJQ5OPE7D) SODIUM CARBONATE 6 [hp_X] in 30 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 2 [hp_X] in 30 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 6 [hp_X] in 30 mL FRANGULA ALNUS BARK (UNII: S2D77IH61R) (FRANGULA ALNUS BARK - UNII:S2D77IH61R) FRANGULA ALNUS BARK 2 [hp_X] in 30 mL RHUBARB (UNII: G280W4MW6E) (RHUBARB - UNII:G280W4MW6E) RHUBARB 2 [hp_X] in 30 mL SKATOLE (UNII: 9W945B5H7R) (SKATOLE - UNII:9W945B5H7R) SKATOLE 6 [hp_X] in 30 mL TARAXACUM OFFICINALE (UNII: 39981FM375) (TARAXACUM OFFICINALE - UNII:39981FM375) TARAXACUM OFFICINALE 2 [hp_X] in 30 mL THIAMINE (UNII: X66NSO3N35) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 4 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-470-18 1 in 1 BOX 01/17/2021 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/17/2021 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-470)