Label: SUCRALFATE tablet
- NDC Code(s): 80425-0091-1
- Packager: Advanced Rx Pharmacy of Tennessee, LLC
- This is a repackaged label.
- Source NDC Code(s): 0093-2210
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 11, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Description
Sucralfate, USP is an α-D-glucopyranoside, β-D-fructofuranosyl-, octakis(hydrogen sulfate), aluminum complex.
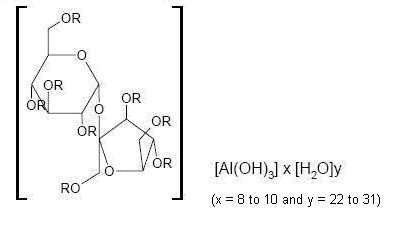
R = SO3Al(OH)2
Tablets for oral administration contain 1 g of sucralfate, USP and the following inactive ingredients: corn starch, magnesium stearate, and microcrystalline cellulose.
Therapeutic Category
antiulcer
-
Clinical Pharmacology
Sucralfate is only minimally absorbed from the gastrointestinal tract. The small amounts of the sulfated disaccharide that are absorbed are excreted primarily in the urine.
Although the mechanism of sucralfate’s ability to accelerate healing of duodenal ulcers remains to be fully defined, it is known that it exerts its effect through a local, rather than systemic, action. The following observations also appear pertinent:
- Studies in human subjects and with animal models of ulcer disease have shown that sucralfate forms an ulcer-adherent complex with proteinaceous exudate at the ulcer site.
- In vitro, a sucralfate-albumin film provides a barrier to diffusion of hydrogen ions.
- In human subjects, sucralfate given in doses recommended for ulcer therapy inhibits pepsin activity in gastric juice by 32%.
- In vitro, sucralfate adsorbs bile salts.
These observations suggest that sucralfate’s antiulcer activity is the result of formation of an ulcer-adherent complex that covers the ulcer site and protects it against further attack by acid, pepsin, and bile salts. There are approximately 14 to 16 mEq of acid-neutralizing capacity per 1 g dose of sucralfate.
-
Clinical Trials
Acute Duodenal Ulcer
Over 600 patients have participated in well-controlled clinical trials worldwide. Multicenter trials conducted in the United States, both of them placebo-controlled studies with endoscopic evaluation at 2 and 4 weeks, showed:
STUDY 1
Treatment Groups
Ulcer Healing/No. Patients
2 wk
4 wk (Overall)
Sucralfate
37/105 (35.2%)
82/109 (75.2%)
Placebo
26/106 (24.5%)
68/107 (63.6%)
STUDY 2
Treatment Groups
Ulcer Healing/No. Patients
2 wk
4 wk (Overall)
Sucralfate
8/24 (33%)
22/24 (92%)
Placebo
4/31 (13%)
18/31 (58%)
The sucralfate-placebo differences were statistically significant in both studies at 4 weeks but not at 2 weeks. The poorer result in the first study may have occurred because sucralfate was given 2 hours after meals and at bedtime rather than 1 hour before meals and at bedtime, the regimen used in international studies and in the second United States study. In addition, in the first study liquid antacid was utilized as needed, whereas in the second study antacid tablets were used.
Maintenance Therapy After Healing of Duodenal Ulcer
Two double-blind randomized placebo-controlled U.S. multicenter trials have demonstrated that sucralfate (1 g bid) is effective as maintenance therapy following healing of duodenal ulcers.
In one study, endoscopies were performed monthly for 4 months. Of the 254 patients who enrolled, 239 were analyzed in the intention-to-treat life table analysis presented below.
Duodenal Ulcer Recurrence Rate (%)
Months of Therapy
Drug
n
1
2
3
4
Sucralfate
122
20 *
30 *
38 †
42†
Placebo
117
33
46
55
63
* p < 0.05
† p < 0.01In this study, prn antacids were not permitted.
In the other study, scheduled endoscopies were performed at 6 and 12 months, but for-cause endoscopies were permitted as symptoms dictated. Median symptom scores between the sucralfate and placebo groups were not significantly different. A life table intention-to-treat analysis for the 94 patients enrolled in the trial had the following results:
Duodenal Ulcer Recurrence Rate (%)
Drug
n
6 months
12 months
Sucralfate
48
19 *
27 *
Placebo
46
54
65
* p < 0.002
In this study, prn antacids were permitted.
Data from placebo-controlled studies longer than 1 year are not available.
-
Indications and Usage Section
INDICATIONS AND USAGE
Sucralfate tablets, USP are indicated in:
Short-term treatment (up to 8 weeks) of active duodenal ulcer. While healing with sucralfate may occur during the first week or two, treatment should be continued for 4 to 8 weeks unless healing has been demonstrated by x-ray or endoscopic examination.
Maintenance therapy for duodenal ulcer patients at reduced dosage after healing of acute ulcers. - Contraindications
-
Precautions
The physician should read the PRECAUTIONS section when considering the use of this drug in pregnant or pediatric patients, or patients of childbearing potential.
Duodenal ulcer is a chronic, recurrent disease. While short-term treatment with sucralfate can result in complete healing of the ulcer, a successful course of treatment with sucralfate should not be expected to alter the post healing frequency or severity of duodenal ulceration.
Isolated reports of sucralfate tablet aspiration with accompanying respiratory complications have been received. Therefore, sucralfate tablets should be used with caution by patients who have known conditions that may impair swallowing, such as recent or prolonged intubation, tracheostomy, prior history of aspiration, dysphagia, or any other conditions that may alter gag and cough reflexes, or diminish oropharyngeal coordination or motility.
Special Populations
Chronic Renal Failure and Dialysis Patients
When sucralfate is administered orally, small amounts of aluminum are absorbed from the gastrointestinal tract. Concomitant use of sucralfate with other products that contain aluminum, such as aluminum-containing antacids, may increase the total body burden of aluminum. Patients with normal renal function receiving the recommended doses of sucralfate and aluminum-containing products adequately excrete aluminum in the urine. Patients with chronic renal failure or those receiving dialysis have impaired excretion of absorbed aluminum. In addition, aluminum does not cross dialysis membranes because it is bound to albumin and transferrin plasma proteins. Aluminum accumulation and toxicity (aluminum osteodystrophy, osteomalacia, encephalopathy) have been described in patients with renal impairment. Sucralfate should be used with caution in patients with chronic renal failure.
Drug Interactions
Some studies have shown that simultaneous sucralfate administration in healthy volunteers reduced the extent of absorption (bioavailability) of single doses of the following: cimetidine, digoxin, fluoroquinolone antibiotics, ketoconazole, l-thyroxine, phenytoin, quinidine, ranitidine, tetracycline, and theophylline. Subtherapeutic prothrombin times with concomitant warfarin and sucralfate therapy have been reported in spontaneous and published case reports. However, two clinical studies have demonstrated no change in either serum warfarin concentration or prothrombin time with the addition of sucralfate to chronic warfarin therapy.
The mechanism of these interactions appears to be nonsystemic in nature, presumably resulting from sucralfate binding to the concomitant agent in the gastrointestinal tract. In all case studies to date (cimetidine, ciprofloxacin, digoxin, norfloxacin, ofloxacin, and ranitidine), dosing the concomitant medication 2 hours before sucralfate eliminated the interaction. Because of the potential of sucralfate to alter the absorption of some drugs, sucralfate should be administered separately from other drugs when alterations in bioavailability are felt to be critical. In these cases, patients should be monitored appropriately.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Chronic oral toxicity studies of 24 months’ duration were conducted in mice and rats at doses up to 1 g/kg (12 times the human dose).
There was no evidence of drug-related tumorigenicity. A reproduction study in rats at doses up to 38 times the human dose did not reveal any indication of fertility impairment. Mutagenicity studies were not conducted.
Pregnancy
Teratogenic Effects
Pregnancy category B
Teratogenicity studies have been performed in mice, rats, and rabbits at doses up to 50 times the human dose and have revealed no evidence of harm to the fetus due to sucralfate. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when sucralfate is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Clinical studies of sucralfate tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy (see DOSAGE AND ADMINISTRATION).
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function (see PRECAUTIONS, Special Populations, Chronic Renal Failure and Dialysis Patients). Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
Adverse Reactions
Adverse reactions to sucralfate in clinical trials were minor and only rarely led to discontinuation of the drug. In studies involving over 2700 patients treated with sucralfate tablets, adverse effects were reported in 129 (4.7%).
Constipation was the most frequent complaint (2%). Other adverse effects reported in less than 0.5% of the patients are listed below by body system:
Gastrointestinal
diarrhea, nausea, vomiting, gastric discomfort, indigestion, flatulence, dry mouth
Dermatological
pruritus, rash
Nervous System
dizziness, insomnia, sleepiness, vertigo
Other
back pain, headache
Postmarketing cases of hypersensitivity have been reported with the use of sucralfate tablets, including dyspnea, lip swelling, pruritus, rash, and urticaria.
Cases of anaphylactic reactions, bronchospasm, laryngeal edema, edema of the mouth, pharyngeal edema, respiratory tract edema and swelling of the face have been reported with an unknown oral formulation of sucralfate.
Bezoars have been reported in patients treated with sucralfate. The majority of patients had underlying medical conditions that may predispose to bezoar formation (such as delayed gastric emptying) or were receiving concomitant enteral tube feedings.
Inadvertent injection of insoluble sucralfate and its insoluble excipients has led to fatal complications, including pulmonary and cerebral emboli. Sucralfate is not intended for intravenous administration.
-
Overdosage
Due to limited experience in humans with overdosage of sucralfate, no specific treatment recommendations can be given. Acute oral toxicity studies in animals, however, using doses up to 12 g/kg body weight, could not find a lethal dose. Sucralfate is only minimally absorbed from the gastrointestinal tract. Risks associated with acute overdosage should, therefore, be minimal. In rare reports describing sucralfate overdose, most patients remained asymptomatic. Those few reports where adverse events were described included symptoms of dyspepsia, abdominal pain, nausea, and vomiting
-
Dosage and Administration Section
DOSAGE AND ADMINISTRATION
Active Duodenal Ulcer
The recommended adult oral dosage for duodenal ulcer is 1 g four times per day on an empty stomach.
Antacids may be prescribed as needed for relief of pain but should not be taken within one-half hour before or after sucralfate.
While healing with sucralfate may occur during the first week or two, treatment should be continued for 4 to 8 weeks unless healing has been demonstrated by x-ray or endoscopic examination.
Maintenance Therapy
The recommended adult oral dosage is 1 g twice a day.
Elderly
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy (see PRECAUTIONS, Geriatric Use).
Call your doctor for medical advice about side effects. You may report side effects to TEVA USA, PHARMACOVIGILANCE at 1-866-832-8537 or drug.safety@tevapharm.com; or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
How Supplied/Storage and Handling
Sucralfate tablets, USP are available as white, capsule-shaped, biconvex, scored tablets, debossed “TEVA” on one side, and “22” and “10” on the scored side, containing 1 gram of sucralfate USP, packaged in bottles of:
Bottles of 30 Tablets NDC 80425-0091-01
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Manufactured In Croatia By:
PLIVA HRVATSKA d.o.o.
Zagreb, Croatia
Manufactured For:
TEVA PHARMACEUTICALS USA, INC.
North Wales, PA 19454
Distributed by:
Advanced Rx Pharmacy of Tennessee LLC, Nashville, TN 37211
Rev. L 9/2015
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SUCRALFATE
sucralfate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:80425-0091(NDC:0093-2210) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SUCRALFATE (UNII: XX73205DH5) (SUCRALFATE - UNII:XX73205DH5) SUCRALFATE 1 g Product Characteristics Color white Score 2 pieces Shape OVAL Size 19mm Flavor Imprint Code TEVA;22;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80425-0091-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 11/11/1996 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA070848 11/11/1996 Labeler - Advanced Rx Pharmacy of Tennessee, LLC (117023142) Establishment Name Address ID/FEI Business Operations Advanced Rx Pharmacy of Tennessee, LLC 117023142 repack(80425-0091)


