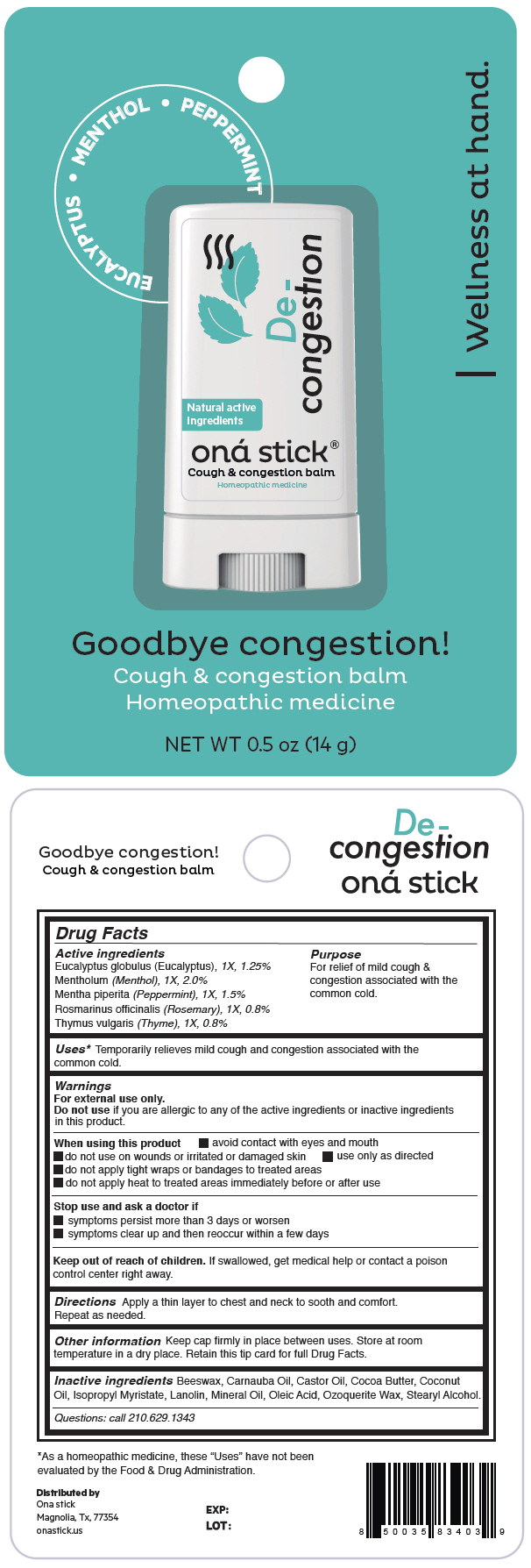
Label: DE-CONGESTION- eucalyptus globulus leaf, menthol, unspecified form, peppermint, rosemary and thymus vulgaris leaf stick
-
Contains inactivated NDC Code(s)
NDC Code(s): 82610-004-14 - Packager: ONA STICK USA LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 17, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses1
Temporarily relieves mild cough and congestion associated with the common cold.
- 1
- As a homeopathic medicine, these "Uses" have not been evaluated by the Food & Drug Administration.
-
Warnings
For external use only.
Do not use if you are allergic to any of the active ingredients or inactive ingredients in this product.
When using this product
- avoid contact with eyes and mouth
- do not use on wounds or irritated or damaged skin
- use only as directed
- do not apply tight wraps or bandages to treated areas
- do not apply heat to treated areas immediately before or after use
- Directions
- Other information
- Inactive ingredients
- Questions
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 14 g Tube Blister Pack
-
INGREDIENTS AND APPEARANCE
DE-CONGESTION
eucalyptus globulus leaf, menthol, unspecified form, peppermint, rosemary and thymus vulgaris leaf stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82610-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) (EUCALYPTUS GLOBULUS LEAF - UNII:S546YLW6E6) EUCALYPTUS GLOBULUS LEAF 1 [hp_X] in 14 g MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 1 [hp_X] in 14 g PEPPERMINT (UNII: V95R5KMY2B) (PEPPERMINT - UNII:V95R5KMY2B) PEPPERMINT 1 [hp_X] in 14 g ROSEMARY (UNII: IJ67X351P9) (ROSEMARY - UNII:IJ67X351P9) ROSEMARY 1 [hp_X] in 14 g THYMUS VULGARIS LEAF (UNII: GRX3499643) (THYMUS VULGARIS LEAF - UNII:GRX3499643) THYMUS VULGARIS LEAF 1 [hp_X] in 14 g Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) CARNAUBA WAX (UNII: R12CBM0EIZ) Castor Oil (UNII: D5340Y2I9G) Cocoa Butter (UNII: 512OYT1CRR) Coconut Oil (UNII: Q9L0O73W7L) Isopropyl Myristate (UNII: 0RE8K4LNJS) Lanolin (UNII: 7EV65EAW6H) Mineral Oil (UNII: T5L8T28FGP) Oleic Acid (UNII: 2UMI9U37CP) CERESIN (UNII: Q1LS2UJO3A) Stearyl Alcohol (UNII: 2KR89I4H1Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82610-004-14 1 in 1 BLISTER PACK 03/15/2022 1 14 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 03/15/2022 Labeler - ONA STICK USA LLC (118525613) Establishment Name Address ID/FEI Business Operations Abinter Labs LLC 117981053 MANUFACTURE(82610-004)