Label: ATOPALM MAXIMUM STRENGTH ANTI-ITCH- hydrocortisone ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 51141-6010-1 - Packager: NeoPharm Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 9, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
-
Uses
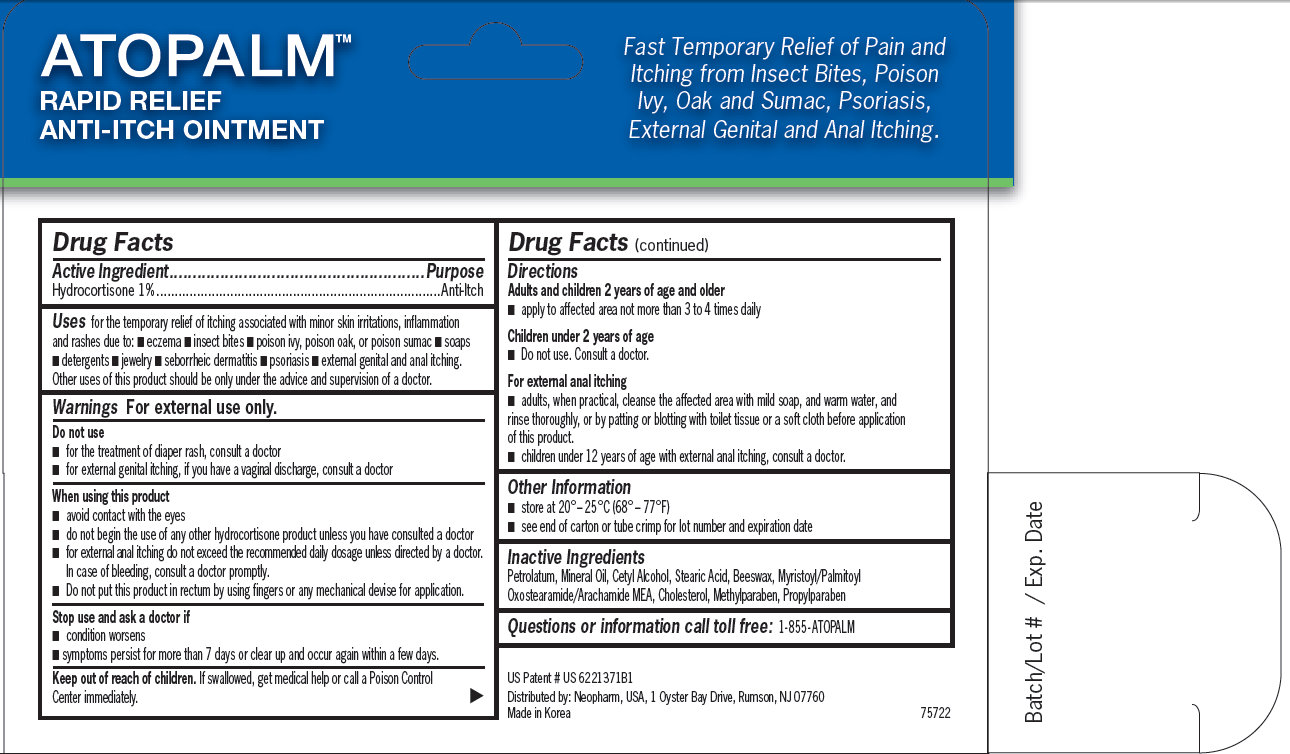
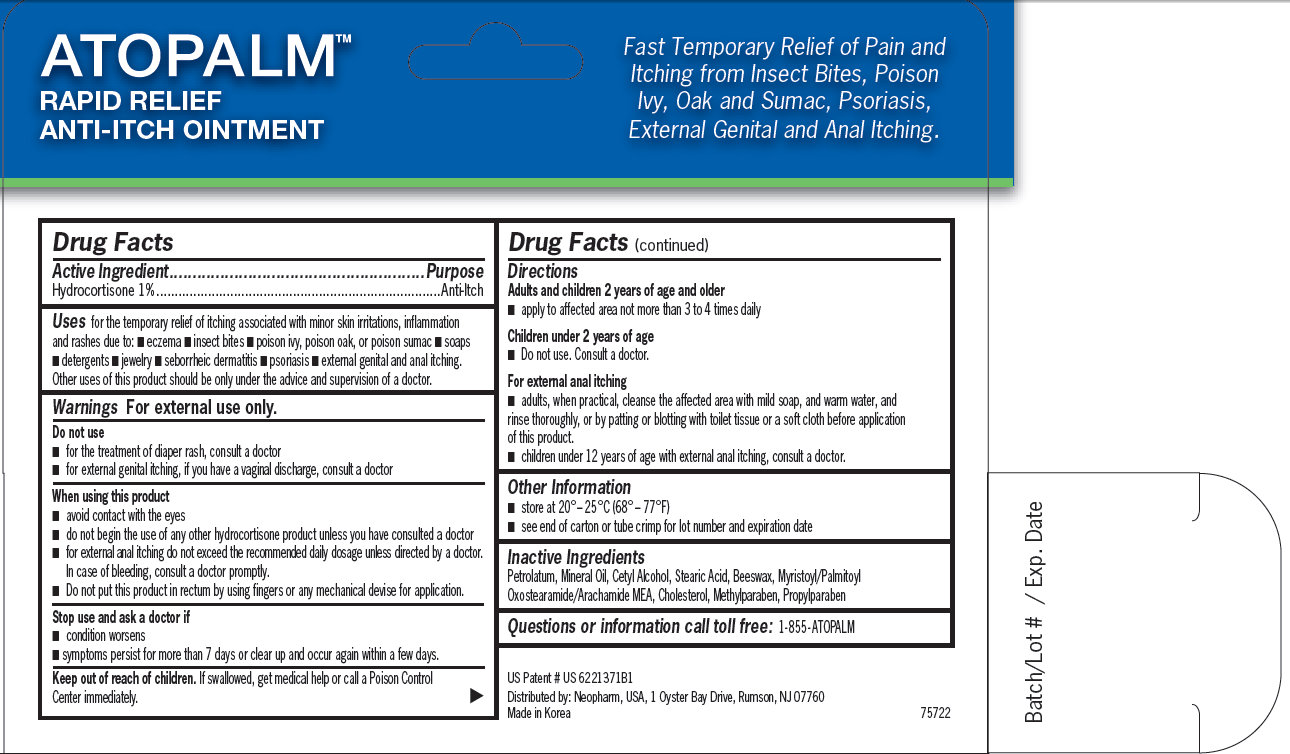
for the temporary relief of itching associated with minor skin irritations, inflammation and rashes due to: - eczema - insect bites - poison ivy, poison oak, or poison sumac - soaps - detergents - jewelry - seborrheic dermatitis - psoriasis - external genital and anal itching. Other uses of this product should be only under the advice and supervision of a doctor.
- Warnings
- Do not use
-
When using this product
- avoid contact with the eyes - do not begin the use of any other hydrocortisone product unless you have consulted a doctor - for external anal itching do not exceed the recommended daily dosage unless directed by a doctor. In case of bleeding, consult a doctor promptly. - Do not put this product in rectum by using fingers or any mechanical devise for application.
- Stop use and ask a doctor if
- Keep out of reach of children
- Other Safety Information
-
Directions
Adults and children 2 years of age and older - apply to affected area not more than 3 to 4 times daily Children under 2 years of age - Do not use. Consult a doctor. For external anal itching - adults, when practical, cleanse the affected area with mild soap, and warm water, and rinse thoroughly, or by patting or blotting with toilet tissue or a soft cloth before application of this product. - children under 12 years of age with external anal itching, consult a doctor.
- Storage & Handling
- Inactive Ingredients
- Questions or information
- Purpose
- Description
-
PRINCIPAL DISPLAY PANEL
Maximum Strength Therapy available without a prescription Anti-Itch Ointment ATOPALM MAXIMUM STRENGTH ANTI-ITCH OINTMENT US Patented M L E Moisturizing Technology Net Wt. 1 oz /28.3 g 1% Hydrocortisone Active Ingredient Hydrocortisone 1% Uses: for the temporary relief of itching associated with minor skin irritations, inflammation and rashes due to eczema, insect bites, poison ivy, poison oak or poison sumac, soaps, detergents, seborrheic dermatitis, psoriasis, external genital and anal itching. Other uses of this product should be only under the advice and supervision of a doctor. Warnings For external use only. Do not use - For the treatment of diaper rash, consult a doctor - For external genital itching, if you have a vaginal discharge, consult a doctor. When using this product - avoid contact with eyes. Do not begin the use of any other hydrocortisone product unless you have consulted a doctor - for external anal itching do not exceed the recommended daily dosage unless directed by a doctor. In case of bleeding, consult a doctor promptly - do not put this product into the rectum by using fingers or any mechanical devise or applicator. Stop use and ask a doctor if - condition worsens - symptoms persist for more than 7 days or clear up and occur again with a few days. Keep out of reach of children. If swallowed, get medical help or call a Poison Control Center immediately. Directions: Adults and children 2 years of age or older - apply to affected area not more than 3 to 4 times daily. Children under 2 years of age - Do not use. Consult a doctor. For external anal itching: - adults, when practical, cleanse the affected area with mild soap, and warm water and rinse thoroughly, or by patting or blotting with toilet tissue or a soft cloth before application of this product. Children under 12 years of age with external anal itching, consult a doctor. Other information: store at 20° – 25°C (68° – 77°F) See end of carton or tube crimp for lot number and expiration date. Questions or information call toll free: 1-855 ATOPALM Distributed by: Neopharm, USA, 1 Oyster Bay Drive, Rumson, NJ 07760 Made in Korea #75722
-
PRINCIPAL DISPLAY PANEL
Maximum Strength Therapy without a prescription 1% Hydrocortisone Anti-Itch Ointment ATOPALM MAXIMUM STRENGTH ANTI-ITCH OINTMENT US Patented M L E Moisturizing Technology Fast, temporary relief of pain, itching and redness from insect bites and skin irritations associated with Poison Ivy, Oak, Sumac, Eczema and Psoriasis. Leaves Skin Looking Visibly Healthier, Softer, Smoother. Maximum Strength without a prescription ATOPALM RAPID RELIEF Rapid Relief of Itching and Redness
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
ATOPALM RAPID RELIEF ANTI-ITCH OINTMENT Fast Temporary Relief of Pain and Itching from Insect Bites, Poison Ivy, Oak and Sumac, Psoriasis, External Genital and Anal Itching. US Patent # US 6221371B1 Distributed by: Neopharm, USA, 1 Oyster Bay Drive, Rumson, NJ 07760 Made in Korea 75722 Batch/Lot # / Exp. Date
- Product Package Inner Label
- Product Package Outer Label 1
- Product Package Outer Label 2
- Product Package Outer Label 3
-
INGREDIENTS AND APPEARANCE
ATOPALM MAXIMUM STRENGTH ANTI-ITCH
hydrocortisone ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51141-6010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 100 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) MINERAL OIL (UNII: T5L8T28FGP) CETYL ALCOHOL (UNII: 936JST6JCN) STEARIC ACID (UNII: 4ELV7Z65AP) YELLOW WAX (UNII: 2ZA36H0S2V) MYRISTOYL/PALMITOYL OXOSTEARAMIDE/ARACHAMIDE MEA (UNII: 1211AIM8G7) CHOLESTEROL (UNII: 97C5T2UQ7J) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51141-6010-1 1 in 1 CARTON 1 28.3 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part348 11/04/2011 Labeler - NeoPharm Co., Ltd. (965502912) Establishment Name Address ID/FEI Business Operations NeoPharm Co., Ltd. 631101883 manufacture