Label: ATROVENTHFA- ipratropium bromide aerosol, metered
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-5511-0 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0597-0087
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 28, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
The active ingredient in ATROVENT HFA Inhalation Aerosol is ipratropium bromide (as the monohydrate). It is an anticholinergic bronchodilator chemically described as 8-azoniabicyclo[3.2.1]octane, 3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-(1-methylethyl)-,bromide monohydrate, (3-endo, 8-syn)-: a synthetic quaternary ammonium compound, chemically related to atropine. The structural formula for ipratropium bromide is:
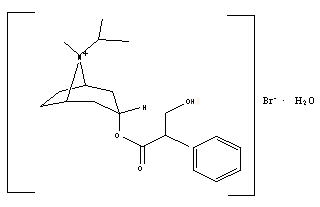
C20H30BrNO3•H2O ipratropium bromide Mol. Wt. 430.4
Ipratropium bromide is a white to off-white crystalline substance, freely soluble in water and methanol, sparingly soluble in ethanol, and insoluble in lipophilic solvents such as ether, chloroform, and fluorocarbons.
ATROVENT HFA Inhalation Aerosol is a pressurized metered-dose aerosol unit for oral inhalation that contains a solution of ipratropium bromide. The 200 inhalation unit has a net weight of 12.9 grams. After priming, each actuation of the inhaler delivers 21 mcg of ipratropium bromide from the valve in 56 mg of solution and delivers 17 mcg of ipratropium bromide from the mouthpiece. The actual amount of drug delivered to the lung may depend on patient factors, such as the coordination between the actuation of the device and inspiration through the delivery system. The excipients are HFA-134a (1,1,1,2-tetrafluoroethane) as propellant, sterile water, dehydrated alcohol, and anhydrous citric acid. This product does not contain chlorofluorocarbons (CFCs) as propellants.
Atrovent® HFA (ipratropium bromide HFA) Inhalation Aerosol should be primed before using for the first time by releasing 2 test sprays into the air away from the face. In cases where the inhaler has not been used for more than 3 days, prime the inhaler again by releasing 2 test sprays into the air away from the face.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Ipratropium bromide is an anticholinergic (parasympatholytic) agent which, based on animal studies, appears to inhibit vagally-mediated reflexes by antagonizing the action of acetylcholine, the transmitter agent released at the neuromuscular junctions in the lung. Anticholinergics prevent the increases in intracellular concentration of Ca++ which is caused by interaction of acetylcholine with the muscarinic receptors on bronchial smooth muscle.
Pharmacodynamic Properties
Controlled clinical studies have demonstrated that Atrovent® (ipratropium bromide) Inhalation Aerosol CFC does not alter either mucociliary clearance or the volume or viscosity of respiratory secretions.
Pharmacokinetics
Most of an administered dose is swallowed as shown by fecal excretion studies. Ipratropium bromide is a quaternary amine. It is not readily absorbed into the systemic circulation either from the surface of the lung or from the gastrointestinal tract as confirmed by blood level and renal excretion studies.
Autoradiographic studies in rats have shown that ipratropium bromide does not penetrate the blood-brain barrier. The half-life of elimination is about 2 hours after inhalation or intravenous administration. Ipratropium bromide is minimally bound (0 to 9% in vitro) to plasma albumin and α1-acid glycoprotein. It is partially metabolized to inactive ester hydrolysis products. Following intravenous administration, approximately one-half of the dose is excreted unchanged in the urine.
A pharmacokinetic study with 29 chronic obstructive pulmonary disease (COPD) patients (48-79 years of age) demonstrated that mean peak plasma ipratropium concentrations of 59±20 pg/mL were obtained following a single administration of 4 inhalations of ATROVENT HFA Inhalation Aerosol (84 mcg). Plasma ipratropium concentrations rapidly declined to 24±15 pg/mL by six hours. When these patients were administered 4 inhalations QID (16 inhalations/day=336 mcg) for one week, the mean peak plasma ipratropium concentration increased to 82±39 pg/mL with a trough (6 hour) concentration of 28±12 pg/mL at steady state.
Special Populations
Geriatric Patients
In the pharmacokinetic study with 29 COPD patients, a subset of 14 patients were > 65 years of age. Mean peak plasma ipratropium concentrations of 56±24 pg/mL were obtained following a single administration of 4 inhalations (21 mcg/puff) of Atrovent® HFA (ipratropium bromide HFA) Inhalation Aerosol (84 mcg). When these 14 patients were administered 4 inhalations QID (16 inhalations/day) for one week, the mean peak plasma ipratropium concentration only increased to 84±50 pg/mL indicating that the pharmacokinetic behavior of ipratropium bromide in the geriatric population is consistent with younger patients.
-
CLINICAL STUDIES
Conclusions regarding the efficacy of ATROVENT HFA Inhalation Aerosol were derived from two randomized, double-blind, controlled clinical studies. These studies enrolled males and females ages 40 years and older, with a history of COPD, a smoking history of > 10 pack- years, an FEV1 < 65% and an FEV1/FVC < 70%.
One of the studies was a 12-week randomized, double-blind active and placebo controlled study in which 505 of the 507 randomized COPD patients were evaluated for the safety and efficacy of 42 mcg (n=124) and 84 mcg (n=126) ATROVENT HFA Inhalation Aerosol in comparison to 42 mcg (n=127) Atrovent® (ipratropium bromide) Inhalation Aerosol CFC and their respective placebos (HFA n=62, CFC n=66). Data for both placebo HFA and placebo CFC were combined in the evaluation.
Serial FEV1 (shown in Figure 1, below, as means adjusted for center and baseline effects on test day 1 and test day 85 (primary endpoint)) demonstrated that 1 dose (2 inhalations/21 mcg each) of ATROVENT HFA Inhalation Aerosol produced significantly greater improvement in pulmonary function than placebo. During the six hours immediately post-dose on day 1, the average hourly improvement in adjusted mean FEV1 was 0.148 liters for ATROVENT HFA Inhalation Aerosol (42 mcg) and 0.013 liters for placebo. The mean peak improvement in FEV1, relative to baseline, was 0.295 liters, compared to 0.138 liters for placebo. During the six hours immediately post-dose on day 85, the average hourly improvement in adjusted mean FEV1 was 0.141 liters for ATROVENT HFA Inhalation Aerosol (42 mcg) and 0.014 liters for placebo. The mean peak improvement in FEV1, relative to baseline, was 0.295 liters, compared to 0.140 liters for placebo.
ATROVENT HFA Inhalation Aerosol (42 mcg) was shown to be clinically comparable to ATROVENT Inhalation Aerosol CFC (42 mcg).
Figure 1 Day 1 and Day 85 (Primary Endpoint) Results
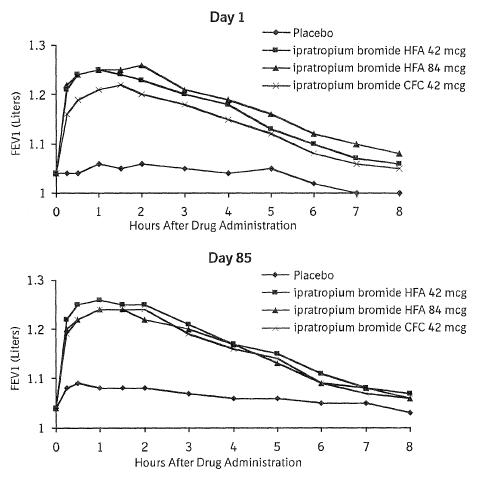
In this study, both Atrovent® HFA (ipratropium bromide HFA) Inhalation Aerosol and Atrovent® (ipratropium bromide) Inhalation Aerosol CFC formulations were equally effective in patients over 65 years of age and under 65 years of age.
The median time to improvement in pulmonary function (FEV1 increase of 15% or more) was within approximately 15 minutes, reached a peak in 1-2 hours, and persisted for 2 to 4 hours in the majority of the patients. Improvements in Forced Vital Capacity (FVC) were also demonstrated.
The other study was a 12-week, randomized, double-blind, active-controlled clinical study in 174 adults with COPD, in which ATROVENT HFA Inhalation Aerosol 42 mcg (n=118) was compared to ATROVENT Inhalation Aerosol CFC 42 mcg (n=56). Safety and efficacy of HFA and CFC formulations were shown to be comparable.
The bronchodilatory efficacy and comparability of Atrovent® HFA (ipratropium bromide HFA) Inhalation Aerosol vs. Atrovent® (ipratropium bromide) Inhalation Aerosol CFC were also studied in a one-year open-label safety and efficacy study in 456 COPD patients. The safety and efficacy of HFA and CFC formulations were shown to be comparable.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
ATROVENT HFA Inhalation Aerosol is a bronchodilator for the maintenance treatment of bronchospasm associated with COPD and is not indicated for the initial treatment of acute episodes of bronchospasm where rescue therapy is required for rapid response.
Immediate hypersensitivity reactions may occur after administration of ipratropium bromide, as demonstrated by urticaria, angioedema, rash, bronchospasm, anaphylaxis, and oropharyngeal edema. If such a reaction occurs, therapy with ATROVENT HFA should be stopped at once and alternative treatment should be considered.
Inhaled medicines, including ATROVENT HFA Inhalation Aerosol, may cause paradoxical bronchospasm. If this occurs, treatment with ATROVENT HFA Inhalation Aerosol should be stopped and other treatments considered.
-
PRECAUTIONS
General
ATROVENT HFA Inhalation Aerosol should be used with caution in patients with narrow-angle glaucoma, prostatic hyperplasia or bladder-neck obstruction.
Information for Patients
Appropriate and safe use of ATROVENT HFA Inhalation Aerosol includes providing the patient with the information listed below and an understanding of the way it should be administered (see Patient's Instructions for Use).
Patients should be advised that ATROVENT HFA Inhalation Aerosol is a bronchodilator for the maintenance treatment of bronchospasm associated with COPD and is not indicated for the initial treatment of acute episodes of bronchospasm where rescue therapy is required for rapid response.
Patients should be cautioned to avoid spraying the aerosol into their eyes and be advised that this may result in precipitation or worsening of narrow-angle glaucoma, mydriasis, increased intraocular pressure, acute eye pain or discomfort, temporary blurring of vision, visual halos or colored images in association with red eyes from conjunctival and corneal congestion. Patients should also be advised that should any combination of these symptoms develop, they should consult their physician immediately.
The action of Atrovent® HFA (ipratropium bromide HFA) Inhalation Aerosol should last 2-4 hours. Patients should be advised not to increase the dose or frequency of ATROVENT HFA Inhalation Aerosol without patients consulting their physician. Patients should also be advised to seek immediate medical attention if treatment with ATROVENT HFA Inhalation Aerosol becomes less effective for symptomatic relief, their symptoms become worse, and/or patients need to use the product more frequently than usual.
Patients should be advised on the use of ATROVENT HFA Inhalation Aerosol in relation to other inhaled drugs.
Patients should be reminded that ATROVENT HFA Inhalation Aerosol should be used consistently as prescribed throughout the course of therapy.
Patients should be advised that although the taste and inhalation sensation of ATROVENT HFA Inhalation Aerosol may be slightly different from that of the CFC (chlorofluorocarbon) formulation of ATROVENT Inhalation Aerosol, they are comparable in terms of safety and efficacy.
Since dizziness, accommodation disorder, mydriasis, and blurred vision may occur with use of ATROVENT, patients should be cautioned about engaging in activities requiring balance and visual acuity such as driving a car or operating appliances, machinery, etc.
Drug Interactions
ATROVENT HFA Inhalation Aerosol has been used concomitantly with other drugs, including sympathomimetic bronchodilators, methylxanthines, oral and inhaled steroids, that may be used in the treatment of chronic obstructive pulmonary disease. With the exception of albuterol, there are no formal studies fully evaluating the interaction effects of ATROVENT and these drugs with respect to effectiveness.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year oral carcinogenicity studies in rats and mice have revealed no carcinogenic activity at doses up to 6 mg/kg (approximately 240 and 120 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis). Results of various mutagenicity studies (Ames test, mouse dominant lethal test, mouse micronucleus test and chromosome aberration of bone marrow in Chinese hamsters) were negative.
Fertility of male or female rats at oral doses up to 50 mg/kg (approximately 2000 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis) was unaffected by ipratropium bromide administration. At an oral dose of 500 mg/kg (approximately 20,000 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis), ipratropium bromide produced a decrease in the conception rate.
Pregnancy
Teratogenic Effects: Pregnancy Category B.
Oral reproduction studies were performed at doses of 10 mg/kg/day in mice, 1,000 mg/kg in rats and 125 mg/kg/day in rabbits. These doses correspond in each species, respectively, to approximately 200, 40000, and 10000 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis. Inhalation reproduction studies were conducted in rats and rabbits at doses of 1.5 and 1.8 mg/kg (approximately 60 and 140 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis). These studies demonstrated no evidence of teratogenic effects as a result of ipratropium bromide. At oral doses 90 mg/kg and above in rats (approximately 3600 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis) embryotoxicity was observed as increased resorption. This effect is not considered relevant to human use due to the large doses at which it was observed and the difference in route of administration. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, Atrovent® HFA (ipratropium bromide HFA) Inhalation Aerosol should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether the active component, ipratropium bromide, is excreted in human milk. Because lipid-insoluble quaternary cations pass into breast milk, caution should be exercised when ATROVENT HFA Inhalation Aerosol is administered to a nursing mother.
Geriatric Use
In the pivotal 12-week study, both ATROVENT HFA Inhalation Aerosol and Atrovent® (ipratropium bromide) Inhalation Aerosol CFC formulations were equally effective in patients over 65 years of age and under 65 years of age.
Of the total number of subjects in clinical studies of ATROVENT HFA Inhalation Aerosol, 57% were ≥ 65 years of age. No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
-
ADVERSE REACTIONS
The adverse reaction information concerning ATROVENT HFA Inhalation Aerosol is derived from two 12-week, double-blind, parallel group studies and one open-label, parallel group study that compared ATROVENT HFA Inhalation Aerosol, ATROVENT Inhalation Aerosol CFC, and placebo (in one study only) in 1,010 COPD patients. The following table lists the incidence of adverse events that occurred at a rate of greater than or equal to 3% in any ipratropium bromide group. Overall, the incidence and nature of the adverse events reported for ATROVENT HFA Inhalation Aerosol, ATROVENT Inhalation Aerosol CFC, and placebo were comparable.
TABLE 1 Adverse Experiences Reported in ≥ 3% of Patients in any Ipratropium Bromide Group Placebo-controlled 12 week Study 244.1405 and
Active-controlled 12 week Study 244.1408
Active-controlled 1-year Study 244.2453 Atrovent
HFA
(N=243)
%Atrovent
CFC
(N=183)
%Placebo
(N=128)
%Atrovent
HFA
(N=305)
%Atrovent
CFC
(N=151)
%Total With Any Adverse Event 63 68 72 91 87 BODY AS A WHOLE - GENERAL DISORDERS Back pain 2 3 2 7 3 Headache 6 9 8 7 5 Influenza-like symptoms 4 2 2 8 5 CENTRAL & PERIPHERAL NERVOUS SYSTEM DISORDERS Dizziness 3 3 2 3 1 GASTROINTESTINAL SYSTEM DISORDERS Dyspepsia 1 3 1 5 3 Mouth dry 4 2 2 2 3 Nausea 4 1 2 4 4 RESPIRATORY SYSTEM DISORDERS Bronchitis 10 11 6 23 19 COPD exacerbation 8 14 13 23 23 Coughing 3 4 6 5 5 Dyspnea 8 8 4 7 4 Rhinitis 4 2 4 6 2 Sinusitis 1 4 3 11 14 Upper respiratory tract infection 9 10 16 34 34 URINARY SYSTEM DISORDERS Urinary tract infection 2 3 1 10 8 In the one open label controlled study in 456 COPD patients, the overall incidence of adverse events was also similar between Atrovent® HFA (ipratropium bromide HFA) Inhalation Aerosol and Atrovent® (ipratropium bromide) Inhalation Aerosol CFC formulations.
Overall, in the above mentioned studies, 9.3% of the patients taking 42 mcg ATROVENT HFA Inhalation Aerosol and 8.7% of the patients taking 42 mcg ATROVENT Inhalation Aerosol CFC reported at least one adverse event that was considered by the investigator to be related to the study drug. The most common drug-related adverse events were dry mouth (1.6% of ATROVENT HFA Inhalation Aerosol and 0.9% of ATROVENT Inhalation Aerosol CFC patients), and taste perversion (bitter taste) (0.9% of ATROVENT HFA Inhalation Aerosol and 0.3% of ATROVENT Inhalation Aerosol CFC patients).
As an anticholinergic drug, cases of precipitation or worsening of narrow-angle glaucoma, glaucoma, halo vision, conjunctival hyperaemia, corneal edema, mydriasis, acute eye pain, dry throat, hypotension, palpitations, urinary retention, tachycardia, constipation, bronchospasm, including paradoxical bronchospasm have been reported. Additional cases identified for ATROVENT seen in clinical trials include throat irritation, stomatitis, mouth edema, and vision blurred.
Allergic-type reactions such as skin rash, pruritus, angioedema including that of tongue, lips and face, urticaria (including giant urticaria), laryngospasm and anaphylactic reactions have been reported (see CONTRAINDICATIONS).
Post-Marketing Experience
In a 5-year placebo-controlled trial, hospitalizations for supraventricular tachycardia and/or atrial fibrillation occurred with an incidence rate of 0.5% in COPD patients receiving ATROVENT Inhalation Aerosol CFC.
Allergic-type reactions such as skin rash, angioedema including that of tongue, lips and face, urticaria (including giant urticaria), laryngospasm and anaphylactic reactions have been reported, with positive rechallenge in some cases.
Additionally, urinary retention, mydriasis, gastrointestinal distress (diarrhea, nausea, vomiting), and bronchospasm, including paradoxical bronchospasm, hypersensitivity reactions, intraocular pressure increased, accommodation disorder, heart rate increased, pharyngeal edema, and gastrointestinal motility disorders have been reported during the post-marketing period with use of ATROVENT.
-
OVERDOSAGE
Acute overdose by inhalation is unlikely since ipratropium bromide is not well absorbed systemically after inhalation or oral administration. Oral median lethal doses of ipratropium bromide were greater than 1001 mg/kg in mice (approximately 20,000 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis); 1,663 mg/kg in rats (approximately 66,000 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis); and 400 mg/kg in dogs (approximately 53,000 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis).
-
DOSAGE AND ADMINISTRATION
Patients should be instructed on the proper use of their inhaler (see Patient's Instructions for Use).
Patients should be advised that although Atrovent® HFA (ipratropium bromide HFA) Inhalation Aerosol may have a slightly different taste and inhalation sensation than that of an inhaler containing Atrovent® (ipratropium bromide) Inhalation Aerosol CFC, they are comparable in terms of the safety and efficacy.
ATROVENT HFA Inhalation Aerosol is a solution aerosol that does not require shaking. However, as with any other metered dose inhaler, some coordination is required between actuating the canister and inhaling the medication.
Patients should "prime" or actuate ATROVENT HFA Inhalation Aerosol before using for the first time by releasing 2 test sprays into the air away from the face. In cases where the inhaler has not been used for more than 3 days, prime the inhaler again by releasing 2 test sprays into the air away from the face. Patients should avoid spraying ATROVENT HFA Inhalation Aerosol into their eyes.
The usual starting dose of ATROVENT HFA Inhalation Aerosol is two inhalations four times a day. Patients may take additional inhalations as required; however, the total number of inhalations should not exceed 12 in 24 hours. Each actuation of ATROVENT HFA Inhalation Aerosol delivers 17 mcg of ipratropium bromide from the mouthpiece.
-
HOW SUPPLIED
ATROVENT HFA Inhalation Aerosol is supplied in a 12.9 g pressurized stainless steel canister as a metered-dose inhaler with a white mouthpiece that has a clear, colorless sleeve and a green protective cap (NDC 54868-5511-0).
The ATROVENT HFA Inhalation Aerosol canister is to be used only with the accompanying ATROVENT HFA Inhalation Aerosol mouthpiece. This mouthpiece should not be used with other aerosol medications. Similarly, the canister should not be used with other mouthpieces. Each actuation of ATROVENT HFA Inhalation Aerosol delivers 21 mcg of ipratropium bromide from the valve and 17 mcg from the mouthpiece. Each 12.9 gram canister provides sufficient medication for 200 actuations. The canister should be discarded after the labeled number of actuations has been used. The amount of medication in each actuation cannot be assured after this point, even though the canister is not completely empty.
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature]. For optimal results, the canister should be at room temperature before use.
Address medical inquiries to: http://us.boehringer-ingelheim.com, (800) 542-6257 or (800) 459-9906 TTY.
Patients should be reminded to read and follow the accompanying “Patient's Instructions for Use”, which should be dispensed with the product.
Contents Under Pressure: Do not puncture. Do not use or store near heat or open flame. Exposure to temperatures above 120°F may cause bursting. Never throw the inhaler into a fire or incinerator.
Warning: Keep out of children's reach. Avoid spraying in eyes.
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USALicensed from:
Boehringer Ingelheim International GmbHCopyright 2010 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVEDRev: July 2010
IT1902GG1710
10003001/06
Relabeling of "Additional Barcode Label" by:
Physicians Total Care, Inc.
Tulsa, OK 74146
-
Patient's Instructions for Use
Atrovent® HFA
(ipratropium bromide HFA)
Inhalation AerosolRead complete instructions carefully before using.
Important Points to Remember About Using ATROVENT HFA Inhalation Aerosol
Although ATROVENT HFA Inhalation Aerosol may taste and feel different
when breathed in compared to your Atrovent® (ipratropium
bromide) Inhalation Aerosol CFC inhaler, they contain the same
medicine.You do not have to shake the ATROVENT HFA Inhalation Aerosol canister
before using it.ATROVENT HFA Inhalation Aerosol should be "primed" two times before
taking the first dose from a new inhaler or when the inhaler has not been used for
more than three days. To prime, push the canister against the mouthpiece (see
Figure 1), allowing the medicine to spray into the air. Avoid spraying the
medicine into your eyes while priming ATROVENT HFA Inhalation
Aerosol.Ask your doctor how to use other inhaled medicines with ATROVENT HFA Inhalation Aerosol.
Use ATROVENT HFA Inhalation Aerosol exactly as prescribed by your doctor. Do not change your dose or how often you use ATROVENT HFA Inhalation Aerosol without talking with your doctor. Talk to your doctor if you have questions about your medical condition or your treatment.
Instructions
- Insert the metal canister into the clear end of the mouthpiece (see Figure 1). Make sure the canister is fully and firmly inserted into the mouthpiece. The ATROVENT HFA Inhalation Aerosol canister is for use only with the ATROVENT HFA Inhalation Aerosol mouthpiece. Do not use the ATROVENT HFA Inhalation Aerosol canister with other mouthpieces. This mouthpiece should not be used with other inhaled medicines.
Figure 1
- Remove the green protective dust cap. If the cap is not on the mouthpiece, make sure there is nothing in the mouthpiece before use. For best results, the canister should be at room temperature before use.
-
Breathe out (exhale) deeply through your mouth. Hold the canister upright as shown in Figure 2, between your thumb and first 2 fingers. Put the mouthpiece in your mouth and close your lips. Keep your eyes closed so that no medicine will be sprayed into your eyes. Atrovent® HFA (ipratropium bromide HFA) Inhalation Aerosol can cause blurry vision, narrow-angle glaucoma or worsening of this condition or eye pain if the medicine is sprayed into your eyes.
Figure 2
-
Breathe in (inhale) slowly through your mouth and at the same time firmly press once on the canister against the mouthpiece as shown in Figure 3. Keep breathing in deeply.
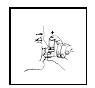
Figure 3
-
Hold your breath for ten seconds and then remove the mouthpiece from your mouth and breathe out slowly, as in Figure 4. Wait at least 15 seconds and repeat steps 3 to 5 again.

Figure 4
- Replace the green protective dust cap after use.
-
Keep the mouthpiece clean. It is very important to keep the mouthpiece clean. At least once a week, wash the mouthpiece, shake it to remove excess water and let it air dry all the way (see the instructions below).
Mouthpiece Cleaning Instructions:
Step A. Remove and set aside the canister and dust cap from the mouthpiece (see Figure 1).
Step B. Wash the mouthpiece through the top and bottom with warm running water for at least 30 seconds (see Figure 5). Do not use anything other than water to wash the mouthpiece.
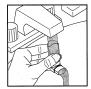
Figure 5
Step C. Dry the mouthpiece by shaking off the excess water and allow it to air-dry all the way.
Step D. When the mouthpiece is dry, replace the canister. Make sure the canister is fully and firmly inserted into the mouthpiece.
Step E. Replace the green protective dust cap.
If the mouthpiece becomes blocked, and little or no medicine comes out of the mouthpiece, wash the mouthpiece as described in Steps A to E under the “Mouthpiece Cleaning Instructions”.
- Keep track of the number of sprays used. Discard the canister after 200 sprays. Even though the canister is not empty, you cannot be sure of the amount of medicine in each spray after 200 sprays.
This product does not contain any chlorofluorocarbon (CFC) propellants.
The contents of Atrovent® HFA (ipratropium bromide HFA) Inhalation Aerosol are under pressure. Do not puncture the canister. Do not use or store near heat or open flame. Exposure to temperatures above 120°F may cause bursting. Never throw the container into a fire or incinerator.
Keep ATROVENT HFA Inhalation Aerosol and all medicines out of the reach of children.
Avoid spraying into eyes.
Address medical inquiries to: http://us.boehringer-ingelheim.com, (800) 542-6257 or (800) 459-9906 TTY.
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). For best results, store the canister at room temperature before use.
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USALicensed from:
Boehringer Ingelheim International GmbHDistributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USALicensed from:
Boehringer Ingelheim International GmbHCopyright 2010 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVEDRev: July 2010
IT1902GG1710
10003001/06 - Insert the metal canister into the clear end of the mouthpiece (see Figure 1). Make sure the canister is fully and firmly inserted into the mouthpiece. The ATROVENT HFA Inhalation Aerosol canister is for use only with the ATROVENT HFA Inhalation Aerosol mouthpiece. Do not use the ATROVENT HFA Inhalation Aerosol canister with other mouthpieces. This mouthpiece should not be used with other inhaled medicines.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ATROVENTHFA
ipratropium bromide aerosol, meteredProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-5511(NDC:0597-0087) Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IPRATROPIUM BROMIDE (UNII: J697UZ2A9J) (IPRATROPIUM BROMIDE - UNII:J697UZ2A9J) IPRATROPIUM BROMIDE 17 ug Inactive Ingredients Ingredient Name Strength NORFLURANE (UNII: DH9E53K1Y8) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-5511-0 1 in 1 CARTON 1 200 in 1 CANISTER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021527 01/20/2006 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel


