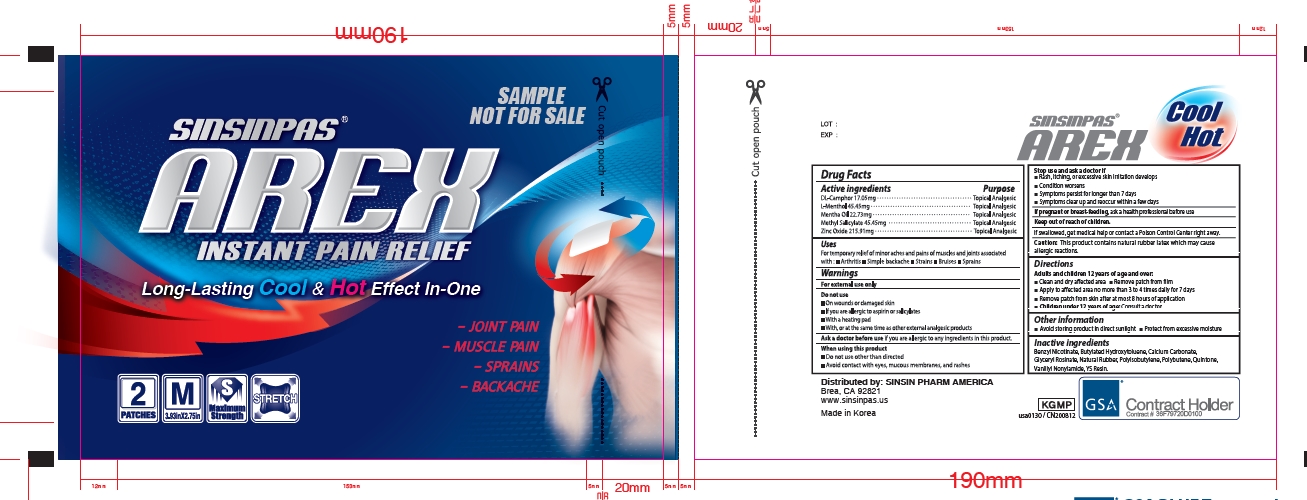
Label: SINSINPAS AREX INSTANT PAIN RELIEF (NOT FOR SALE)- camphor, mentha oil, menthol, methyl salicylate, zinc oxide patch
- NDC Code(s): 55264-108-01, 55264-108-02
- Packager: Sinsin Pharmaceutical Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- WARNINGS AND PRECAUTIONS
- Directions
- Other information
- Inactive ingredients
- Manufactured by:
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SINSINPAS AREX INSTANT PAIN RELIEF (NOT FOR SALE)
camphor, mentha oil, menthol, methyl salicylate, zinc oxide patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55264-108 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 17.05 mg PEPPERMINT OIL (UNII: AV092KU4JH) (PEPPERMINT - UNII:V95R5KMY2B) PEPPERMINT OIL 22.73 mg LEVOMENTHOL (UNII: BZ1R15MTK7) (LEVOMENTHOL - UNII:BZ1R15MTK7) LEVOMENTHOL 45.45 mg METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 45.45 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 215.91 mg Inactive Ingredients Ingredient Name Strength BENZYL NICOTINAMIDE (UNII: HF1W0213QP) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CALCIUM CARBONATE (UNII: H0G9379FGK) GLYCERIN (UNII: PDC6A3C0OX) ROSIN (UNII: 88S87KL877) NATURAL LATEX RUBBER (UNII: 2LQ0UUW8IN) POLYISOBUTYLENE (200000 MW) (UNII: Z6Y02I0591) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) NONIVAMIDE (UNII: S846B891OR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55264-108-02 1 in 1 CARTON 11/06/2020 1 NDC:55264-108-01 2 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/06/2020 Labeler - Sinsin Pharmaceutical Co., Ltd. (823149161) Registrant - Sinsin Pharmaceutical Co., Ltd. (823149161) Establishment Name Address ID/FEI Business Operations Sinsin Pharmaceutical Co., Ltd. 687867143 manufacture(55264-108)