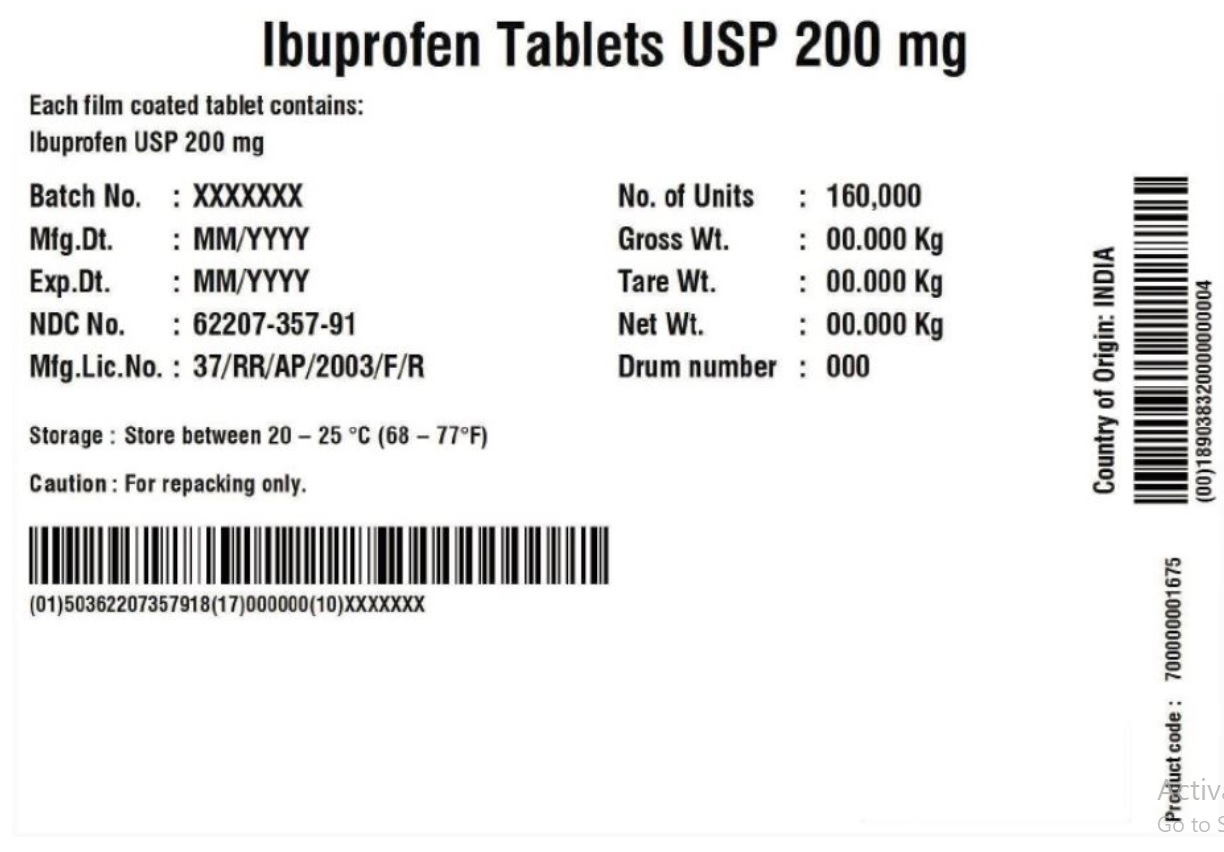
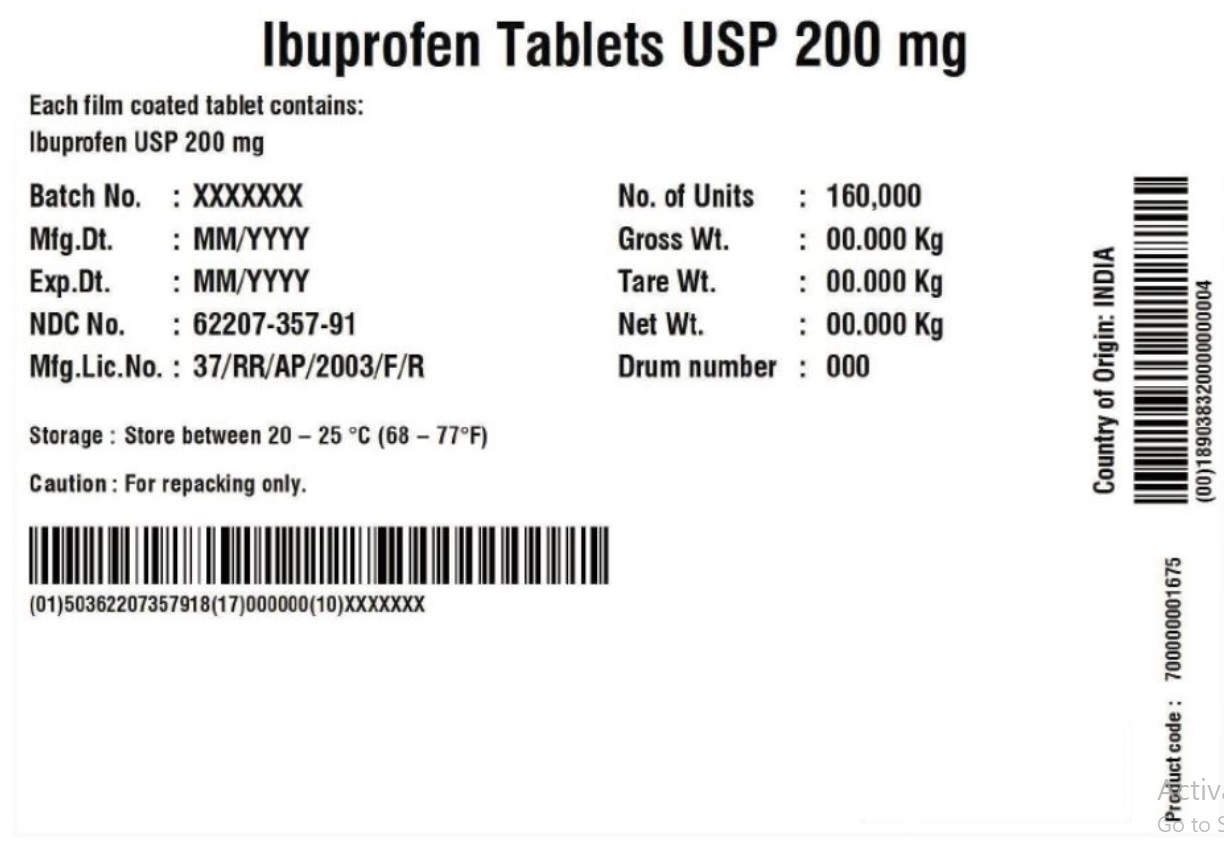
Label: IBUPROFEN tablet
-
NDC Code(s):
62207-357-41,
62207-357-42,
62207-357-43,
62207-357-46, view more62207-357-47, 62207-357-48, 62207-357-49, 62207-357-91, 62207-358-41, 62207-358-42, 62207-358-43, 62207-358-46, 62207-358-47, 62207-358-48, 62207-358-49, 62207-358-91
- Packager: Granules India Limited
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 2, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT(S)
- PURPOSE
- USE(S)
-
WARNINGS
Allergy Alert:
Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin.
Symptoms may include:
- hives
- facial swelling
- asthma(wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning:This product contains an NSAID, which may cause stomach
bleeding. The chances is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning(anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Heart attack and stroke warning: NSAIDs, except aspirin increase the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed
- DO NOT USE
-
ASK A DOCTOR BEFORE USE IF
- you have problems or serious side effects from taking pain relievers or fever reducers
- the stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease, asthma or had a stroke
- you are taking a diuretic
- ASK A DOCTOR OR PHARMACIST BEFORE USE IF YOU ARE
- WHEN USING THIS PRODUCT
-
STOP USE AND ASK DOCTOR IF
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- you have symptoms of heart problems or stroke, chest pain, trouble breathing, weakness in one part or side of body, slurred speech, leg swelling
- pain gets worse or lasts more than10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
- IF PREGNANT OR BREAST-FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
• do not take more than directed
• the smallest effective dose should be used
adults and children
12 years and older
• take 1 tablet/caplet every 4 to 6 hours while symptoms persist.
• if pain or fever does not respond to 1 tablet/caplet, 2 tablets/caplets may be
used.
• do not exceed 6 tablets/caplets in 24 hours, unless directed by a doctor.
children under 12 years
• ask a doctor
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS OR COMMENTS?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IBUPROFEN
ibuprofen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62207-357 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) POVIDONE K30 (UNII: U725QWY32X) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color orange Score no score Shape ROUND Size 10mm Flavor Imprint Code G;3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62207-357-41 24 in 1 BOTTLE; Type 0: Not a Combination Product 11/17/2016 2 NDC:62207-357-42 50 in 1 BOTTLE; Type 0: Not a Combination Product 11/17/2016 3 NDC:62207-357-43 100 in 1 BOTTLE; Type 0: Not a Combination Product 11/17/2016 4 NDC:62207-357-46 250 in 1 BOTTLE; Type 0: Not a Combination Product 11/17/2016 5 NDC:62207-357-47 500 in 1 BOTTLE; Type 0: Not a Combination Product 11/17/2016 6 NDC:62207-357-48 750 in 1 BOTTLE; Type 0: Not a Combination Product 11/17/2016 7 NDC:62207-357-49 1000 in 1 BOTTLE; Type 0: Not a Combination Product 11/17/2016 8 NDC:62207-357-91 160000 in 1 DRUM 10/08/2018 8 1 in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202312 11/17/2016 IBUPROFEN
ibuprofen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62207-358 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) POVIDONE K30 (UNII: U725QWY32X) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color orange Score no score Shape OVAL Size 14mm Flavor Imprint Code G;3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62207-358-41 24 in 1 BOTTLE; Type 0: Not a Combination Product 11/17/2016 2 NDC:62207-358-42 50 in 1 BOTTLE; Type 0: Not a Combination Product 11/17/2016 3 NDC:62207-358-43 100 in 1 BOTTLE; Type 0: Not a Combination Product 11/17/2016 4 NDC:62207-358-46 250 in 1 BOTTLE; Type 0: Not a Combination Product 11/17/2016 5 NDC:62207-358-47 500 in 1 BOTTLE; Type 0: Not a Combination Product 11/17/2016 6 NDC:62207-358-48 750 in 1 BOTTLE; Type 0: Not a Combination Product 11/17/2016 7 NDC:62207-358-49 1000 in 1 BOTTLE; Type 0: Not a Combination Product 11/17/2016 8 NDC:62207-358-91 160000 in 1 DRUM 11/17/2016 8 1 in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202312 11/17/2016 Labeler - Granules India Limited (915000087) Registrant - Granules India Limited (915000087) Establishment Name Address ID/FEI Business Operations Granules India Ltd 918609236 manufacture(62207-357, 62207-358) , label(62207-357, 62207-358)