Label: VIZAMYL- flutemetamol f-18 solution
- NDC Code(s): 17156-067-10, 17156-067-30
- Packager: Medi-Physics, Inc. dba GE Healthcare
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VIZAMYL safely and effectively. See full prescribing information for VIZAMYL.
VIZAMYL (flutemetamol F 18 injection) for intravenous use
Initial U.S. Approval: 2013INDICATIONS AND USAGE
Vizamyl is a radioactive diagnostic agent indicated for Positron Emission Tomography (PET) imaging of the brain to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer's disease (AD) or other causes of cognitive decline. A negative Vizamyl scan indicates sparse to no neuritic plaques, and is inconsistent with a neuropathological diagnosis of AD at the time of image acquisition; a negative scan result reduces the likelihood that a patient's cognitive impairment is due to AD. A positive Vizamyl scan indicates moderate to frequent amyloid neuritic plaques; neuropathological examination has shown this amount of neuritic plaque is present in patients with AD, but may also be present in patients with other types of neurologic conditions, as well as older people with normal cognition. Vizamyl is an adjunct to other diagnostic evaluations (1).
Limitations of Use:
DOSAGE AND ADMINISTRATION
- Use appropriate radiation safety handling measures (2.1)
- Administer 185 megabecquerels (MBq) [5 millicuries (mCi)] within 40 seconds as a single intravenous bolus in a total volume of 10 mL or less (2.2)
- Follow injection with an intravenous flush of 5 to 15 mL of 0.9% sterile sodium chloride injection (2.2)
- Obtain 10 to 20-minute PET images starting approximately 60 to 120 minutes after intravenous injection (2.3)
- Image interpretation: Refer to full prescribing information (2.4, 2.5)
- The radiation dose absorbed from a 185-MBq (5-mCi) dose of Vizamyl is 5.92 mSv in an adult (2.6)
DOSAGE FORMS AND STRENGTHS
Injection: 150 MBq/mL (4.05 mCi/mL) at reference date and time in 30 mL multi-dose vials (3).
CONTRAINDICATIONS
Known hypersensitivity to Vizamyl or any excipient, including polysorbate 80 (4).
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions: Ask patients about prior reactions to Vizamyl. Observe for hypersensitivity signs and symptoms following Vizamyl administration. Have resuscitation equipment and trained personnel available at time of Vizamyl administration (5.1)
- Image interpretation errors (especially false positives) have been observed (5.2)
- Radiation risk: Vizamyl, similar to all radiopharmaceuticals, contributes to a patient's long-term cumulative radiation exposure. Ensure safe handling to protect patients and health care workers from unintentional radiation exposure (2.1, 5.3)
ADVERSE REACTIONS
Most commonly reported adverse reactions were flushing (2%), headache (1%), increased blood pressure (2%), nausea (1%), and dizziness (1%).
To report SUSPECTED ADVERSE REACTIONS, contact GE Healthcare at 1-800-654-0118 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
USE IN SPECIFIC POPULATIONS
Lactation: A lactating woman may pump and discard breast milk for 24 hours after Vizamyl administration. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety - Drug Handling
2.2 Recommended Dosing and Administration Procedures
2.3 Imaging Acquisition Guidelines
2.4 Image Orientation and Display
2.5 Image Interpretation
2.6 Radiation Dosimetry
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Risk for Image Misinterpretation and Other Errors
5.3 Radiation Risk
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
11.1 Physical Characteristics
11.2 External Radiation
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
16.3 Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Vizamyl is indicated for Positron Emission Tomography (PET) imaging of the brain to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer's Disease (AD) and other causes of cognitive decline.
A negative Vizamyl scan indicates sparse to no neuritic plaques and is inconsistent with a neuropathological diagnosis of AD at the time of image acquisition; a negative scan result reduces the likelihood that a patient's cognitive impairment is due to AD. A positive Vizamyl scan indicates moderate to frequent amyloid neuritic plaques; neuropathological examination has shown this amount of amyloid neuritic plaque is present in patients with AD, but may also be present in patients with other types of neurologic conditions as well as in older people with normal cognition. Vizamyl is an adjunct to other diagnostic evaluations.
Limitations of Use:
- A positive Vizamyl scan does not establish a diagnosis of AD or other cognitive disorder.
- Safety and effectiveness of Vizamyl have not been established for:
- Predicting development of dementia or other neurologic condition.
- Monitoring responses to therapies.
-
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety - Drug Handling
Vizamyl is a radioactive drug and should be handled with safety measures to minimize radiation exposure during administration [see Warnings and Precautions (5.3)]. Use waterproof gloves and effective shielding, including lead-glass syringe shields when handling and administering Vizamyl. To minimize radiation dose to the bladder, encourage patients to hydrate before and after Vizamyl administration in order to permit frequent voiding. Encourage patients to void before and after imaging with Vizamyl and frequently thereafter for 24 hours following Vizamyl administration.
Radiopharmaceuticals, including Vizamyl, should be used by or under the control of physicians who are qualified by specific training and experienced in the safe use and handling of radioactive materials, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radiopharmaceuticals.
2.2 Recommended Dosing and Administration Procedures
The recommended dose for Vizamyl is 185 megabecquerels (MBq) [5 millicuries (mCi)] in a maximum dose volume of 10 mL, administered as a single intravenous bolus within 40 seconds. The maximum mass dose is 20 micrograms. Follow the injection with an intravenous flush of 5 to 15 mL of 0.9% sterile sodium chloride injection.
- Use aseptic technique and radiation shielding to withdraw and administer Vizamyl solution.
- Calculate the necessary volume to administer based on calibration time and dose using a suitably calibrated instrument.
- Visually inspect Vizamyl for particulate matter and discoloration prior to administration. Do not administer Vizamyl if it contains particulate matter or is discolored [see Description (11)].
- Do not dilute Vizamyl.
- Dispose of unused product in a safe manner in compliance with applicable regulations [see How Supplied/Storage and Handling (16)].
2.3 Imaging Acquisition Guidelines
The recommended PET scan start time is 60 to 120 minutes after Vizamyl injection, using a PET scanner in 3-D mode with appropriate data corrections. A scan duration of 10 to 20 minutes is recommended. The time of initiation and the duration of the scan may vary depending on dose, imaging acquisition, and reconstruction parameters. Position the patient supine with the brain (including the cerebellum) within a single field of view. The patient's head should be tilted so that the anterior commissure-posterior commissure (AC-PC) plane is at right angles to the bore-axis of the PET scanner, with the head positioned in a suitable head support. Reducing head movement with tape or other flexible head restraints may be employed. Iterative or filtered back-projection reconstruction is recommended with a slice thickness of 2 to 4 mm, matrix size of 128 × 128 with pixel sizes of approximately 2 mm. Where a post-smoothing filter is applied, a full width half maximum (FWHM) of not more than 5 mm is recommended; filter FWHM should be chosen to optimize the signal-to-noise ratio while preserving the sharpness of the reconstructed image.
2.4 Image Orientation and Display
Image Orientation
Orient axial and coronal images to show symmetry of brain structures, with equal heights of structures bilaterally. Orient sagittal images so that the head and neck are neither flexed nor extended; the anterior and posterior aspects of the corpus callosum should be parallel to the AC-PC line as shown in Figure 2.
Image Display
- Display images with all planes (axial, sagittal and coronal planes) linked by crosshairs.
- Select a color scale that provides a progression of low through high intensity (e.g., rainbow or Sokoloff). The selected color scale should (1) provide colors that allow the reader to discriminate intensity levels above and below the intensity level of the pons, (2) provide a color for regions with little or no amyloid binding such as the cerebellar cortex, and (3) provide a range of at least five distinct colors above 50 to 60% of the peak intensity.
- Display the reference scale. Adjust the color scale to set the pons to approximately 90% maximum intensity. The cerebellar cortex should represent approximately 20-30% of peak intensity on both negative and positive Vizamyl scans.
- Briefly display axial brain slices from bottom to top and look for signs of atrophy.
- Systematically review the following brain regions (recommended plane) for flutemetamol F 18 uptake as described in Image Interpretation below:
- Frontal lobes (axial, with optional sagittal plane view)
- Posterior cingulate and precuneus (sagittal, with optional coronal plane view)
- Lateral temporal lobes (axial, with optional coronal plane view)
- Inferolateral parietal lobes (coronal, with optional axial plane view)
- Striatum (axial, with optional sagittal plane view)
2.5 Image Interpretation
Vizamyl images should be interpreted only by readers who successfully complete the electronic or in-person training program provided by the manufacturer [see Warnings and Precautions (5.2)]. The objective of Vizamyl image interpretation is to provide an estimate of the brain β-amyloid neuritic plaque density, not to make a clinical diagnosis. Image interpretation is performed independently of a patient's clinical features and relies upon recognition of image features in certain brain regions.
Image interpretation is based upon the distribution of radioactive signal within the brain; clinical information is not a component of image assessment [see Warnings and Precautions (5.2)]. Images are designated as positive or negative either by comparing radioactivity in cortical grey matter with activity in adjacent white matter, or based on the intensity in the five regions mentioned above. Signal uptake in the cerebellum does not contribute to scan interpretation (for example, a positive scan may show retained cerebellar grey-white contrast even when the cortical grey-white contrast is lost). Images should be viewed with the minimum image intensity set to zero and the maximum set such that the signal level in the easily identifiable pons is at 90% of maximum.
Negative scans show more radioactivity in white matter than in grey matter, creating clear grey-white matter contrast.
Specifically, a negative scan would have the following characteristics:
- frontal, lateral temporal, inferolateral parietal lobes: gradual gradient from bright intensity of the white matter to lower intensity at the periphery of the brain; distinct sulci with concave surfaces (white matter sulcal pattern),
-
and -
posterior cingulate and precuneus: grey matter uptake below 50-60% of peak intensity; gap of lower intensity separates two hemispheres on coronal view, -
and -
striatum: approximately 50% of peak intensity or lower in the region between the higher intensities of the thalamus and frontal white matter (striatal "gap")
Positive scans show at least one cortical region with reduction or loss of the normally distinct grey-white matter contrast. These scans have one or more regions with increased cortical grey matter signal (above 50-60% peak intensity) and/or reduced (or absent) grey-white matter contrast (white matter sulcal pattern is less distinct). A positive scan may have one or more regions in which grey matter radioactivity is as intense or exceeds the intensity in adjacent white matter.
Specifically, a positive scan would have the following characteristics:
- frontal, lateral temporal, or inferolateral parietal lobes: high intensity seen to the periphery of the brain, with sharp reduction of intensity at the brain margin; sulci not distinct due to fill-in by high intensity grey matter resulting in a convex surface at the edge of the brain,
-
or -
posterior cingulate and precuneus: grey matter uptake above 50-60% of peak intensity; high grey matter intensity that closes the gap between the two hemispheres on coronal view, -
or -
striatum: intensity above 50-60% of peak intensity; gap between thalamus and frontal white matter not distinct -
If any one of the brain regions systematically reviewed for flutemetamol F 18 uptake (see Image Orientation and Display above) is positive in either hemisphere, then the scan is considered positive. Otherwise, the scan is considered negative.
Among patients with clinically important β-amyloid neuritic plaques in the brain, the temporal lobes, parietal lobes, and striatum may not be as affected compared to other brain regions. Therefore, in some images, flutemetamol F 18 signal in these regions may not be as intense as in the frontal lobes or the posterior cingulate and precuneus regions.
Atrophy may affect the interpretability of scans, particularly in the frontal, temporal and parietal lobes [see Warnings and Precautions (5.2)]. For cases in which atrophy is apparent or suspected and there is uncertainty as to the location of the grey matter on the PET scan, examine the striatum for flutemetamol F 18 signal as it is less affected by atrophy than other regions of the brain.
If the patient's MRI or CT brain images are available the interpreter should examine the CT or MRI images to clarify the relationship between PET flutemetamol F 18 uptake and grey matter anatomy.
Other factors that may affect the ability to interpret Vizamyl images include patient factors such as brain pathology, surgical changes, post-radiation therapy changes, and implants. Some scans may be difficult to interpret due to image noise, suboptimal patient positioning, or over-smoothing of the reconstructed image.
Figure 1: Axial view of negative (left) and positive (right) Vizamyl scans. The axial slices which cut through the frontal pole and inferior aspect of the splenium are shown using a rainbow color scale. The left image shows a white matter sulcal pattern at the frontal (f) and lateral temporal (lt) regions with a color intensity that tapers to the periphery, as well as less radioactivity in the striatal region(s). The right image shows absence of the white matter sulcal pattern with intensity radiating to a sharply defined convex edge, as well as more radioactivity in the striatum. In both the frontal and lateral temporal regions, the intensity is higher in the grey matter regions of the right image compared to those of the left image.
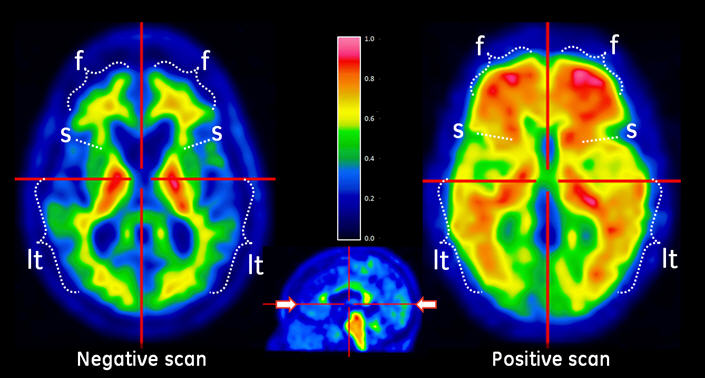
Figure 2: Sagittal view of negative (left) and positive (right) Vizamyl scans. The sagittal slices are slightly off midline in one hemisphere and shown using a rainbow color scale. In the posterior cingulate (pc) region, which is superior and posterior to the corpus callosum (cc), the left image shows intensity below 50% of peak intensity whereas the right image shows intensity above 60% of peak intensity. The pons (p) is set to approximately 90% of the maximum intensity.
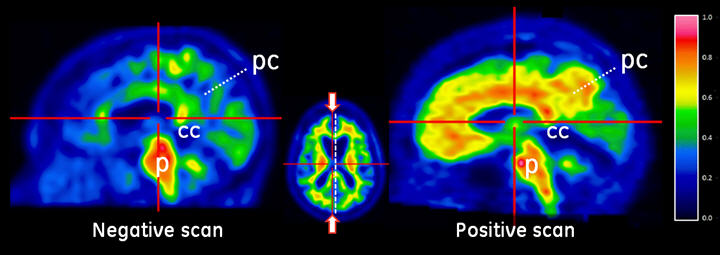
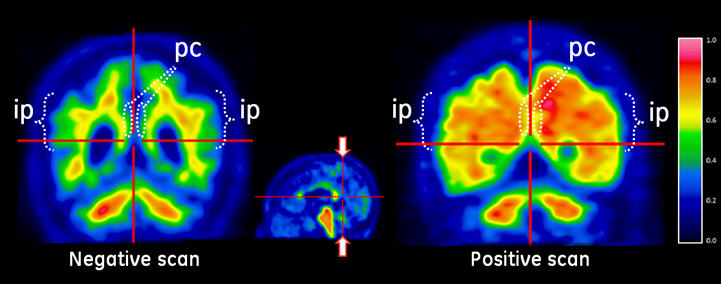
Figure 3: Coronal view of negative (left) and positive (right) Vizamyl scans. The coronal slices are located posterior to the corpus callosum. The left image shows a white matter sulcal pattern in the inferior parietal (ip) regions that is not evident in the right image. Relative to the left image, the right image shows increased intensity in the posterior cinguli (pc) and increased radial extent of high intensity to the lateral surfaces of the parietal lobes particularly evident in the inferior parietal regions.
2.6 Radiation Dosimetry
The estimated absorbed radiation doses for adult patients following intravenous injection of Vizamyl are shown in Table 1. Values were calculated from human biodistribution data using OLINDA/EXM software and assuming emptying of the urinary bladder at 3.5 hour intervals.
The adult effective dose resulting from a 185-MBq (5-mCi) Vizamyl administration is 5.92 mSv. The use of a CT scan to calculate attenuation correction for reconstruction of Vizamyl images (as done in PET/CT imaging) will add radiation exposure at the level of approximately 0.1 mSv effective dose. Diagnostic head CT scans using helical scanners administer an average of 2.2 ± 1.3 mSv effective dose. The actual radiation dose is operator and scanner dependent.
Table 1: Adult Estimated Radiation Absorbed Vizamyl Doses in Organs/Tissues Organ/Tissue Absorbed Radiation Dose Per Unit Administered Activity
microGy/MBqAdrenals 13 Brain 11 Breasts 5 Gallbladder wall 287 Heart wall 14 Kidneys 31 Liver 57 Lower large intestine wall 42 Lungs 16 Muscle 9 Osteogenic cells 11 Ovaries 25 Pancreas 15 Red marrow 13 Skin 5 Small intestine wall 102 Spleen 15 Stomach wall 12 Testes 8 Thymus 6 Thyroid 6 Upper large intestine wall 117 Urinary bladder wall 145 Uterus 25 Total body 12 Effective Dose 32 (microSv/MBq) - 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Vizamyl is contraindicated in patients with a history of hypersensitivity reaction to Vizamyl, polysorbate 80, or any other inactive ingredient in Vizamyl [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions such as flushing and dyspnea have been observed within minutes following Vizamyl administration. These reactions may occur in patients with no history of prior exposure to Vizamyl.
Before administering Vizamyl, ask patients about prior reactions to drugs, especially those containing polysorbate 80.
Have resuscitation equipment and trained personnel immediately available at the time of Vizamyl administration [see Contraindications (4)].
5.2 Risk for Image Misinterpretation and Other Errors
Errors may occur while using Vizamyl PET images to estimate brain neuritic plaque density [see Clinical Studies (14)].
Image interpretation is performed independently of the patient's clinical information. The use of clinical information in the interpretation of Vizamyl images has not been evaluated and may lead to errors. Extensive brain atrophy may limit the ability to distinguish grey and white matter on a Vizamyl scan [see Dosage and Administration (2.5)]. Motion artifacts may distort the image [see Dosage and Administration (2.3)].
Vizamyl scan results are indicative of the brain neuritic amyloid plaque content only at the time of image acquisition and a negative scan result does not preclude the development of brain amyloid in the future.
5.3 Radiation Risk
Vizamyl, similar to other radiopharmaceuticals, contributes to a patient's overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk of cancer. Ensure safe handling to protect patients and health care workers from unintentional radiation exposure [see Dosage and Administration (2.1)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Clinical trials are conducted under widely varying conditions and adverse reaction rates observed in the clinical trials of Vizamyl cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In clinical trials, 761 adults (367 men and 394 women, 91% Caucasian) with a mean age of 62 years (range 18-93 years) received Vizamyl. Most subjects (530, 70%) received a dose of 185 MBq (5 mCi).
One subject out of 761 administered Vizamyl experienced a serious hypersensitivity reaction with flushing, dyspnea and chest pressure within minutes following Vizamyl administration and recovered with treatment.
Most adverse reactions were mild to moderate in intensity and resolved spontaneously. The most commonly reported adverse reactions (occurring in at least 1% of subjects) in Vizamyl-treated subjects are shown in Table 2.
Table 2: Adverse Reactions Reported in Clinical Trials of Vizamyl (N = 761 subjects) Adverse Reaction N (percent of patients) Flushing 16 (2%) Increased blood pressure 13 (2%) Headache 10 (1%) Nausea 8 (1%) Dizziness 8 (1%) -
7 DRUG INTERACTIONS
Pharmacodynamic drug-drug interaction studies have not been performed in patients to establish the extent, if any, to which concomitant medications may alter Vizamyl image results.
Within a clinical study of patients with a range of cognitive impairment, some patients were receiving the following medications: donepezil, galantamine, memantine, rivastigmine. Mean cortical Standardized Uptake Value (SUV) ratios did not differ between the patients taking or not taking these concomitant medications.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on Vizamyl use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. All radiopharmaceuticals, including Vizamyl, have the potential to cause fetal harm depending on the stage of fetal development, and the magnitude of the radiopharmaceutical dose. If considering Vizamyl administration to a pregnant woman, inform the patient about the potential for adverse pregnancy outcomes based on the radiation dose from Vizamyl and the gestational timing of exposure.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of Flutemetamol (F 18) or metabolites in human milk or its effects on the breastfed infant or milk production. Exposure of Vizamyl to a breastfed infant can be minimized by temporary discontinuation of breastfeeding [see Clinical Considerations]. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Vizamyl and any potential adverse effects on the breastfed child from Vizamyl or from the underlying maternal condition.
-
10 OVERDOSAGE
The clinical consequence of overdose with Vizamyl has not been reported. It is unknown whether or not flutemetamol is dialyzable. The major risks of overdose relate predominantly to increased radiation exposure, with long-term risks for neoplasia. In case of overdose of radioactivity, hydration and frequent urination should be encouraged to minimize radiation exposure to the patient; care should be taken to avoid contamination from the radioactivity eliminated by the patient.
-
11 DESCRIPTION
Vizamyl contains flutemetamol F 18, a molecular imaging agent that binds to β-amyloid aggregates, and is intended for use with PET imaging of the brain. Chemically, flutemetamol F 18, is described as 2-[3-[18F]fluoro-4-(methylamino) phenyl]-6-benzothiazolol. It has the molecular formula C14H1118FN2OS, molecular weight 273.32, and the following structural formula:

Vizamyl is a sterile, non-pyrogenic, clear, colorless to slightly yellow radioactive solution for intravenous injection. Each milliliter (mL) of the no-carrier added Vizamyl product solution contains 150 MBq (4.05 mCi) of flutemetamol F 18 at reference date and time and up to 2 micrograms of flutemetamol. Each mL of the Vizamyl solution also contains 70 microliters ethanol, 9.0 mg sodium chloride and 4.98 mg polysorbate 80 (w/v) in 0.014 M aqueous phosphate buffer. The pH of the solution is between 6.0 and 8.5.
11.1 Physical Characteristics
Fluorine-18 (F 18) is a cyclotron-produced radionuclide that decays by positron emission (β+ decay, 96.7%) and orbital electron capture (3.3%) to stable oxygen-18 with a physical half-life of 109.8 minutes. The positron can undergo annihilation with an electron to produce two gamma rays; the energy of each gamma ray is 511 keV (Table 3).
Table 3: Principal Radiation Emission Data – Fluorine-18 Radiation Energy (keV) Abundance (%) Gamma 511 193.4 Positron 249.8 96.7 11.2 External Radiation
The point source air-kerma rate constant for F-18 is 3.74E -17 Gy m2/(Bq s); this coefficient was formerly defined as the specific gamma-ray constant of 5.7 R/hr/mCi at 1 cm. The first half-value thickness of lead (Pb) for F 18 gamma rays is approximately 6 mm. The relative reduction of radiation emitted by F-18 that results from various thicknesses of lead shielding is shown in Table 4. The use of ~8 cm of Pb will decrease the radiation transmission (i.e., exposure) by a factor of about 10,000.
Table 4: Radiation Attenuation of 511 keV Gamma Rays by Lead Shielding Shielding Thickness cm of lead (Pb) Coefficient of Attenuation 0.6 0.5 2 0.1 4 0.01 6 0.001 8 0.0001 -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Flutemetamol F 18 binds to β-amyloid plaques in the brain and the F-18 isotope produces a positron signal that is detected by a PET scanner. In in vitro binding studies using postmortem human brain homogenates containing fibrillar β-amyloid, the dissociation constant (Kd) for flutemetamol was 6.7 nM.
Selectivity of [3H]flutemetamol binding in post-mortem human brain sections was demonstrated using autoradiography, silver-stained protein, and immunohistochemistry (monoclonal antibody to β-amyloid) correlation studies.
12.2 Pharmacodynamics
Following intravenous injection, flutemetamol F 18 diffuses across the human blood-brain barrier and produces a radioactivity signal detectable throughout the brain. Subsequently, cerebral perfusion decreases the brain flutemetamol F 18 content, with differential retention of the drug in cortical areas that contain β-amyloid aggregates compared to areas that lack the aggregates. The time-activity curves for flutemetamol F 18 in the brain of subjects with positive scans shows continual signal increases from time zero through 30 minutes post administration, with stable values thereafter up to at least 120 minutes post-injection. Differences in signal intensity between brain regions that specifically retain flutemetamol F 18 and brain regions with nonspecific retention of the drug form the basis of image interpretation methods [see Dosage and Administration (2.5)].
The test-retest distribution of flutemetamol F 18 was evaluated in 5 subjects with probable AD who underwent two administrations of flutemetamol F 18 (followed by PET scans) separated by a time period of 1 to 4 weeks. Images were reproducible when evaluated semi-quantitatively using an automated assessment of SUV in pre-specified cortical regions of brain.
12.3 Pharmacokinetics
Following intravenous injection of 185 MBq (5 mCi) of Vizamyl in humans, flutemetamol F 18 plasma concentrations declined by approximately 75% in the first 20 minutes post-injection, and by approximately 90% in the first 180 minutes. The F 18 in circulation during the 30-120 minutes imaging window in plasma was principally associated with flutemetamol metabolites. Excretion was approximately 37% renal (28-45%; n=6) and 52% hepatobiliary (40-65%; n=6).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies have not been performed to evaluate the carcinogenicity potential of flutemetamol. Flutemetamol was positive for mutagenicity in two in vitro assays: the bacterial reverse mutation assay (Ames test) and the mouse lymphoma assay.
Flutemetamol was negative for genotoxicity after in vivo exposure in rats to flutemetamol at the highest cumulative dose level tested, as measured in bone marrow micronucleus assays (157 and 27 microgram/kg/day for 2 and 14 days respectively) and an unscheduled DNA synthesis assay in rat hepatocytes (39 microgram/kg/day).
-
14 CLINICAL STUDIES
Vizamyl was evaluated in two clinical studies (Study One and Study Two) in adult subjects with a range of cognitive function, including some terminally ill patients who had agreed to participate in a post-mortem brain donation program. Both studies were single-arm and subjects underwent Vizamyl injection and scan. The images were interpreted by five independent readers masked to all clinical information; readers in Study Two were naïve to all forms of amyloid PET imaging. PET images were reviewed first without, and subsequently with, brain CT or MRI images.
Study One evaluated pre-mortem Vizamyl PET images from terminally ill patients and compared the results to postmortem truth standard assessments of cerebral cortical neuritic plaque density in patients who died during the study. Readers evaluated images using a clinically applicable binary image interpretation method (positive/negative) that involved evaluating regional Vizamyl brain uptake to yield a final overall image assessment that was compared to the truth standard. Before image interpretation, all readers underwent in-person tutoring on image interpretation. To determine the agreement between the in vivo Vizamyl image results and the post-mortem whole brain amyloid neuritic plaque density, Vizamyl results (negative/positive) were pre-specified to correspond with specific global histopathology plaque density scores, based upon a modification of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) criteria (Table 5), which use neuritic plaque counts as a necessary pathological feature of AD. Plaques were counted on microscope slides with modified Bielschowsky silver-stained tissue sections. The global brain neuritic plaque density score for each subject was determined by averaging across the scores (0-3) for five grey matter fields per slide and then across the six slides for each of eight regions; if any one region had a regional score of greater than 1.5, the subject's brain was classified as positive for amyloid.
Table 5: Global and Regional Neuritic Plaque Score Correlates to Vizamyl Image Results Vizamyl Image Result CERAD Classification (Score) Neuritic Plaque Counts Negative None (0) 0 Sparse (1) 1 to 5 Positive Moderate (2) 6 to 19 Frequent (3) >20 In Study One, one hundred eighty patients were dosed with Vizamyl and 176 were imaged. The median patient age was 82 years (range 47 to 98 years) and 57% of the patients were female. By medical history 44 patients had no cognitive impairment, 135 had dementia, no patients had mild cognitive impairment (MCI), and one patient had memory loss of unspecified nature. Sixty-nine patients died during the study; 68 had cerebral cortical amyloid status determined (43 positive and 25 negative) and were included in the primary analysis. The time interval between the Vizamyl scan and death ranged from 0 to 13 months, with a median of 2.6 months, and was less than one year for 66 patients and between 12 to 13 months for 2 patients. At autopsy, the global brain neuritic plaque density category (CERAD classification as in Table 5) was available for 67/68 subjects: frequent (n = 19); moderate (n = 22); sparse (n = 14); and none (n = 12).
In Study Two, the effectiveness of an electronic training program for Vizamyl image orientation and interpretation was evaluated using Vizamyl PET images from across subjects with different cognitive abilities who had participated in earlier studies. Inter-reader reproducibility of image interpretation was assessed using images from subjects with a truth standard (68 patients who underwent an autopsy and 36 known or suspected normal pressure hydrocephalus patients with in vivo brain biopsy) and without a truth standard (28 cognitively normal volunteers 55 years or above, 80 patients with amnestic mild cognitive impairment (aMCI), 33 patients with probable AD (pAD)), and 31 young healthy volunteers. Additionally, intra-reader reproducibility was assessed from 29 images (10%). Among the 276 subjects, the median age was 72 years (range 20 to 95), 136 were females, and 251 were Caucasian.
Vizamyl performance characteristics for Study One and Study Two patients with an autopsy-based truth standard are shown in Table 6 and Table 7. Among patients who underwent autopsy (n=68; 43 positive and 25 negative based on histopathology), the median (and range) of correct read results, false negatives, and false positives were 59 (51, 61), 5 (3, 8), 3 (2, 14), respectively, for in-person training (Study One); and were 60 (55 to 61), 3 (3 to 6), 4 (2 to 10), respectively, for electronic media training (Study Two). Image reproducibility for various subject groups in Study Two is presented in Table 8. Inter-reader reproducibility analysis showed an overall Fleiss' kappa statistic of 0.83 (95% CI 0.79 to 0.86) which met the pre-specified success criterion (95% CI lower bound > 0.60). Intra-reader reproducibility analysis showed that, between the two readings for each of the 29 duplicate patient images, one of the five readers had complete agreement for all 29 images, two readers had discordant reads for a single image, and two readers had discordant reads for two images. Intra-reader reproducibility for a subgroup of 8 images from aMCI patients showed that all five readers had complete agreement for all duplicate images.
Table 6. Vizamyl Scan Results by Reader Training Method among Patients with Autopsy (n = 68) Test Performance In-Person Training
(Study One)Electronic Media Training
(Study Two)Sensitivity (%) Median 88 93 Range among the 5 readers 81 - 93 86 - 93 Specificity (%) Median 88 84 Range among the 5 readers 44 - 92 60 - 92 Table 7: Vizamyl Scan Interpretations by Reader Training Method among Autopsied Patients (n = 68) In-Person Training
(Study One)Electronic Media Training
(Study Two)Reader Reader 1 2 3 4 5 6 7 8 9 10 - *
- 43 positive and 25 negative based on histopathology
All scans with autopsies
(n = 68*)Correct 57 60 51 59 61 58 61 61 55 60 False Negative 8 5 3 3 5 3 3 4 3 6 False Positive 3 2 14 5 2 7 4 3 10 2 Table 8 (Study Two): Median Number of Positive Vizamyl Scans and Reproducibility of Scan Results Subject Group by Cognitive and Truth Standard (TS) Positive Scans
N*Kappa
(95% CI)Percent of Scans with Inter-reader Agreement 3 of 5 readers agreed 4 of 5 readers agreed 5 of 5 readers agreed pAD: probable AD; aMCI: amnestic MCI; Elderly: 55 years or above - *
- Shown is the median number of scans interpreted as positive across the 5 readers for each subgroup of subjects listed in the first column.
- †
- 30 with TS from autopsy
- ‡
- 21 with TS from autopsy, 0 with TS from biopsy
- §
- 17 from autopsy, 5 of 36 with TS from biopsy were not definitively classified as pAD based on clinical diagnosis
All 276 subjects 139 0.83 (0.79, 0.86) 5 14 81 All subjects with a TS, n=104 (68 autopsy; 36 biopsy) 58 0.74 (0.68, 0.80) 6 24 70 All subjects without a TS, n = 172 76 0.88 (0.83, 0.92) 5 8 87 pAD, n = 63 (30 with TS†; 33 no TS) 47 0.88 (0.80, 0.96) 3 6 90 aMCI, n = 80 (0 with TS) 45 0.89 (0.82, 0.96) 4 7 89 Elderly cognitively normal without TS, n = 28 2 0.46 (0.34, 0.57) 4 14 82 Cognitively normal with TS‡, (n=21) 10 0.64 (0.5, 0.77) 5 38 57 Other (non-AD) dementia with TS, n=53§ 27 0.71 (0.63, 0.80) 8 25 68 -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Vizamyl is supplied in a 30-mL multi-dose glass vial with 1-30 mL fill volume. Each vial is enclosed in an appropriate radiation shield. The total concentration is 150 MBq/mL (4.05 mCi/mL) of flutemetamol F 18 at reference date and time.
30-mL sterile multi-dose vial with variable fill volume: NDC 17156-067-30
-
17 PATIENT COUNSELING INFORMATION
Instruct patients to inform their healthcare provider if they:
- are pregnant or breast feeding, or
- have had prior reactions to Vizamyl or any component, including polysorbate 80, or
- have reduced renal or hepatic function
Instruct patients to increase their level of hydration before and after receiving Vizamyl (Flutemetamol F 18 Injection) and to void frequently for the first 24 hours following Vizamyl administration.
Manufactured for GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Vizamyl is a trademark of General Electric Company or one of its subsidiaries.
GE and the GE Monogram are trademarks of General Electric Company.© 2020 General Electric Company – All rights reserved.
43-1067D
-
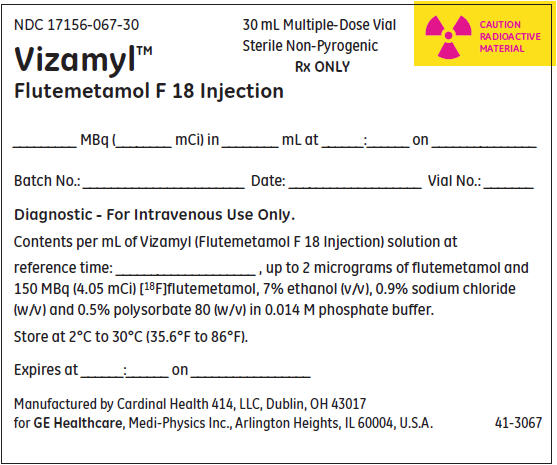
PRINCIPAL DISPLAY PANEL - Shield Label
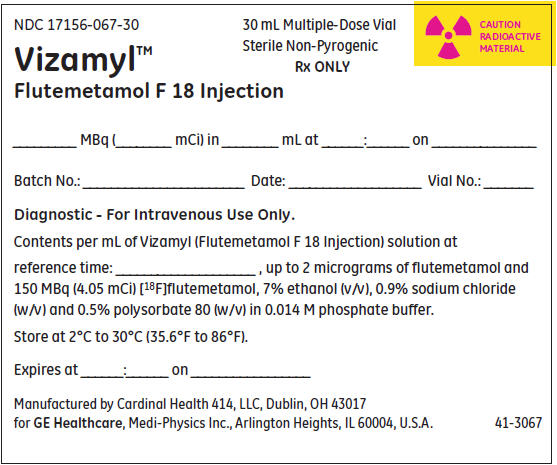
NDC 17156-067-30
Vizamyl™
Flutemetamol F 18 Injection30 mL Multiple-Dose Vial
Sterile Non-Pyrogenic
Rx ONLYCAUTION
RADIOACTIVE
MATERIAL_______ MBq (________ mCi) in ________ mL at ______:______ on _______________
Batch No.: _______________________ Date: ___________________ Vial No.: _______
Diagnostic - For Intravenous Use Only.
Contents per mL of Vizamyl (Flutemetamol F 18 Injection) solution at
reference time: ____________________ , up to 2 micrograms of flutemetamol and
150 MBq (4.05 mCi) [18F]flutemetamol, 7% ethanol (v/v), 0.9% sodium chloride
(w/v) and 0.5% polysorbate 80 (w/v) in 0.014 M phosphate buffer.Store at 2°C to 30°C (35.6°F to 86°F).
Expires at ______:______ on _________________
Manufactured by Cardinal Health 414, LLC, Dublin, OH 43017
for GE Healthcare, Medi-Physics Inc., Arlington Heights, IL 60004, U.S.A.41-3067
-
INGREDIENTS AND APPEARANCE
VIZAMYL
flutemetamol f-18 solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:17156-067 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Flutemetamol F-18 (UNII: L49M066S0O) (Flutemetamol F-18 - UNII:L49M066S0O) Flutemetamol F-18 4.05 mCi in 1 mL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) 70 uL in 1 mL Sodium Chloride (UNII: 451W47IQ8X) Polysorbate 80 (UNII: 6OZP39ZG8H) Product Characteristics Color YELLOW (colorless to slightly yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17156-067-10 1 in 1 CONTAINER 03/01/2014 06/18/2018 1 10 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product 2 NDC:17156-067-30 1 in 1 CONTAINER 03/01/2014 2 30 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203137 01/01/2014 Labeler - Medi-Physics, Inc. dba GE Healthcare (095263729) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (Sacramento, CA) 165486861 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (Phoenix, AZ) 833114734 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) Establishment Name Address ID/FEI Business Operations Precision Nuclear, LLC (Johnson City, TN) 879283633 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) Establishment Name Address ID/FEI Business Operations Cardinal Health, Inc. (East Lansing, MI) 963972166 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (Colton, CA) 964767656 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (Beltsville, MD) 964767771 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (Seattle, WA) 964768126 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (Dallas, TX) 964768282 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (Tampa, FL) 964768340 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) Establishment Name Address ID/FEI Business Operations Cardinal Health 414, LLC (Charlotte, NC) 964768373 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) Establishment Name Address ID/FEI Business Operations Cardinal Health 418, Inc. (Aurora, CO) 149029253 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) Establishment Name Address ID/FEI Business Operations Cardinal Health, Inc. (Houston, TX) 826800364 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) Establishment Name Address ID/FEI Business Operations Cardinal Health, Inc. (Glendale Heights, IL) 033231923 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) Establishment Name Address ID/FEI Business Operations Cardinal Health, Inc. (New Orleans, LA) 080130462 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) Establishment Name Address ID/FEI Business Operations Cardinal Health, Inc. (E. Hartford, CT) 964768233 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) Establishment Name Address ID/FEI Business Operations Cardinal Health, Inc. (Ft. Lauderdale, FL) 964767722 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) , MANUFACTURE(17156-067) Establishment Name Address ID/FEI Business Operations The University of Utah DBA Cyclotron Radiochemistry Lab Huntsman Cancer Institute (Salt Lake City, UT) 018432646 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) Establishment Name Address ID/FEI Business Operations Essential Isotopes, LLC. (Colombia, MO) 010753961 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) Establishment Name Address ID/FEI Business Operations Pharmalogic Colorado, LLC 117608150 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(17156-067) Establishment Name Address ID/FEI Business Operations GE Healthcare AS (Oslo, Norway) 515048908 MANUFACTURE(17156-067) , ANALYSIS(17156-067) Establishment Name Address ID/FEI Business Operations Medi-Physics, Inc. dba GE Healthcare 095263729 ANALYSIS(17156-067)