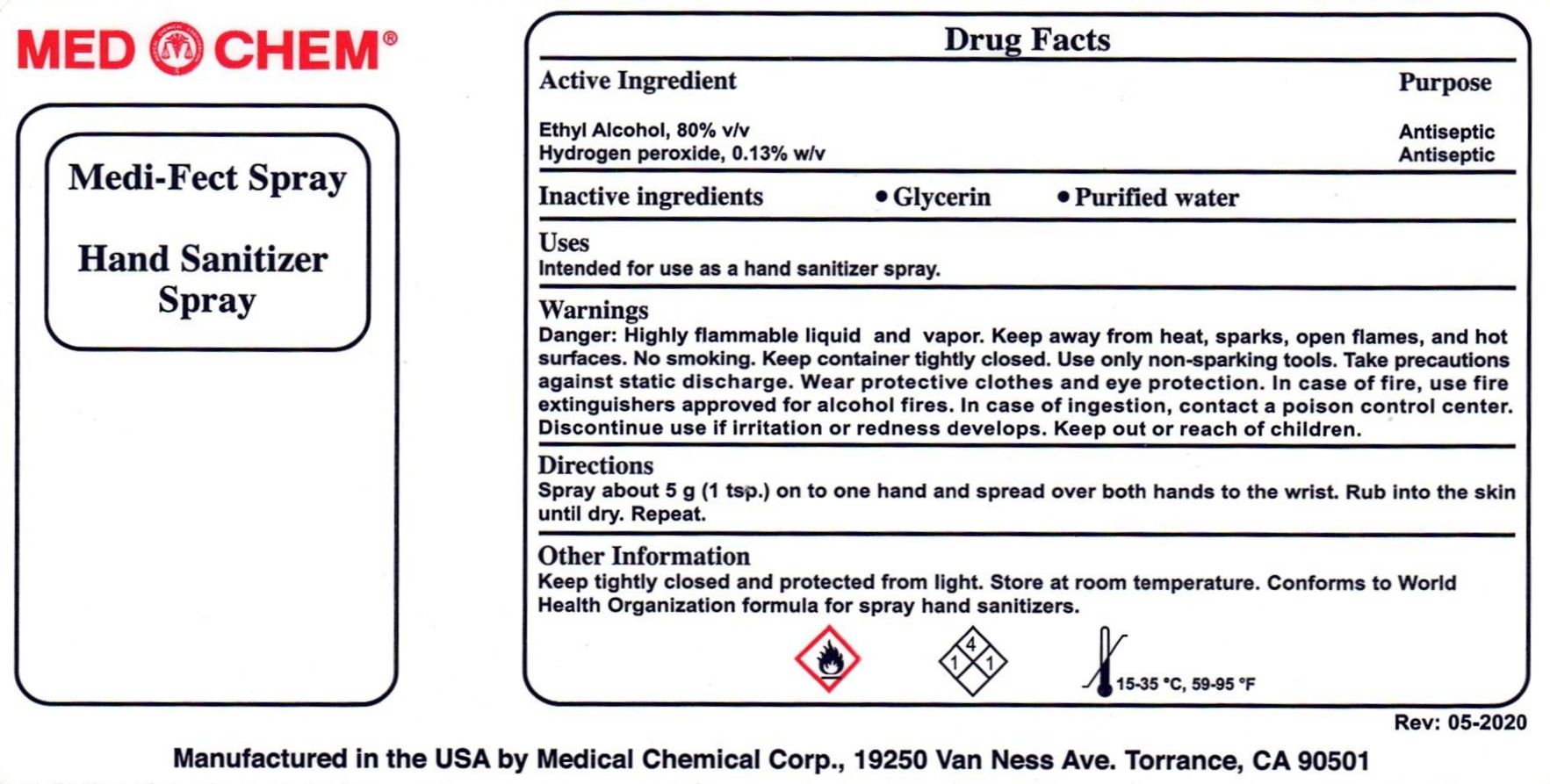
Label: MEDIFECT HAND SANITIZER- alcohol liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 12745-901-01, 12745-901-02, 12745-901-03 - Packager: Medical Chemical Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 17, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
This is a hand sanitizer manufactured according to the Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (CoViD-19); Guidance for Industry.
The hand sanitizer is manufactured using only the following United States Pharmacopoeia (USP) grade ingredients in the preparation of the product (percentage in final product formulation) consistent with World Health Organization (WHO) recommendations:
- Alcohol (ethanol) (USP or Food Chemical Codex (FCC) grade) (80%, volume/volume (v/v)) in an aqueous solution denatured according to Alcohol and Tobacco Tax and Trade Bureau regulations in 27 CFR part 20.
- Glycerol (1.45% v/v).
- Hydrogen peroxide (0.125% v/v).
- Sterile distilled water or boiled cold water.
The firm does not add other active or inactive ingredients. Different or additional ingredients may impact the quality and potency of the product.
- Active Ingredient(s)
- Purpose
- Use
-
Warnings
Danger: Highly flammable liquid and vapor. Keep away from heat, sparks, open flames, and hot surfaces. No smoking. Keep container tightly closed. Use only non-sparking tools. Take precautions against static discharge. Wear protective clothes and eye protection. In case of fire, use fire extinguishers approved for alcohol fires. In case of ingestion, contact a poison control center. Discontinue use if irritation or redness develops. Keep out of reach of children.
- Warnings
- Directions
- Other information
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
MEDIFECT HAND SANITIZER
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:12745-901 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROGEN PEROXIDE (UNII: BBX060AN9V) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) HYDROGEN PEROXIDE 4.17 mL in 100 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 80 mL in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) 1.45 mL in 100 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:12745-901-01 237 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/23/2020 2 NDC:12745-901-02 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/23/2020 3 NDC:12745-901-03 3785 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/23/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 03/30/2020 Labeler - Medical Chemical Corporation (008496861) Registrant - Medical Chemical Corporation (008496861) Establishment Name Address ID/FEI Business Operations Medical Chemical Corporation 008496861 manufacture(12745-901)