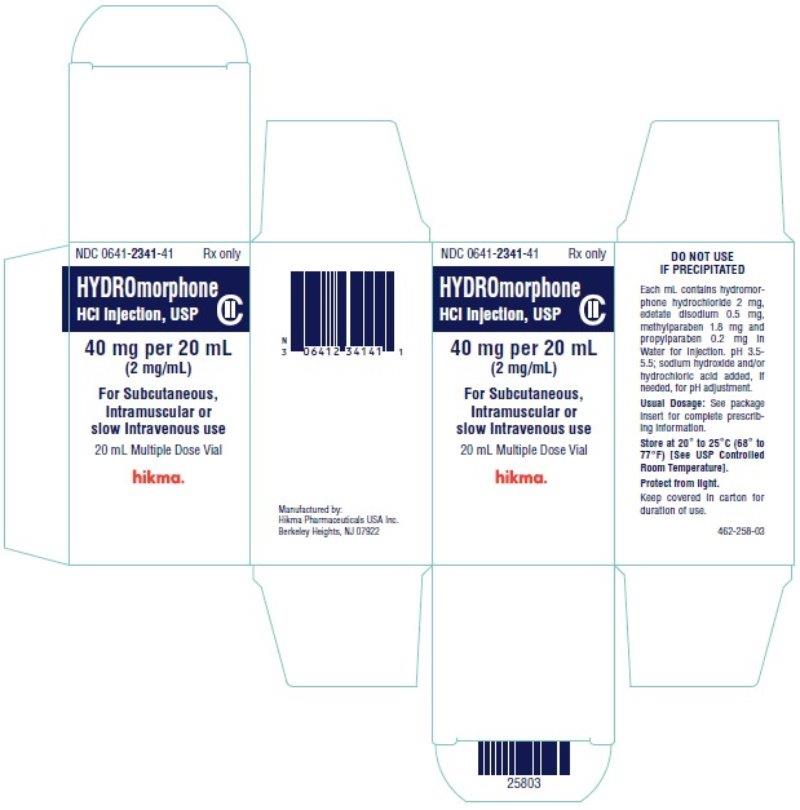
Label: HYDROMORPHONE HYDROCHLORIDE injection
- NDC Code(s): 0641-0121-21, 0641-0121-25, 0641-2341-39, 0641-2341-41
- Packager: Hikma Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Hydromorphone Hydrochloride Injection, USP is a sterile solution intended for subcutaneous, intramuscular or slow intravenous injection. Each mL contains hydromorphone hydrochloride 2 mg, edetate disodium 0.5 mg, methylparaben 1.8 mg and propylparaben 0.2 mg in Water for Injection. The pH range is 3.5-5.5; sodium hydroxide and/or hydrochloric acid added, if needed, for pH adjustment. Hydromorphone is a semisynthetic phenanthrene alkaloid of opium; it is classified pharmacologically as a narcotic analgesic. Hydromorphone hydrochloride may be named chemically as 4,5α-Epoxy-3-hydroxy-17-methylmorphinan-6-one hydrochloride, with the following structural formula:
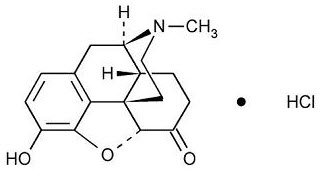
C17H19NO3• HCl M.W. 321.80
Hydromorphone hydrochloride occurs as a fine, white or practically white, crystalline powder and is freely soluble in water and sparingly soluble in alcohol.
-
CLINICAL PHARMACOLOGY
Hydromorphone resembles morphine both structurally and pharmacologically. Like other narcotic analgesics, hydromorphone exerts its principal pharmacological effects on the central nervous system and gastrointestinal tract. Its primary action of therapeutic value is analgesia. The analgesic effects of hydromorphone are due to its central action; however, the precise mechanism of action of hydromorphone and other opiates is not known, although it is believed to relate to the existence of opiate receptors in the central nervous system. Hydromorphone, like other narcotic analgesics, appears to increase the patient’s tolerance for pain and to decrease the perception of suffering, although the presence of the pain itself may still be recognized.
In addition to analgesia, narcotics commonly produce such CNS effects as drowsiness, alterations in mood and mental clouding. Hydromorphone is reported to produce analgesia with less sedation than morphine. This may be an advantage in the postoperative period, since the patient with a less-clouded sensorium is better able to cooperate in early ambulation procedures. Likewise, cancer patients can be relieved of pain yet remain sufficiently alert to function within the scope of their underlying physical disorder. Narcotic analgesics also depress various respiratory centers, depress the cough reflex, constrict the pupils, elevate cerebrospinal fluid pressure, produce transient hyperglycemia and enhance parasympathetic activity.
Narcotic analgesics may cause nausea and vomiting by stimulating the chemoreceptor trigger zone (CTZ); however, they also depress the vomiting center, so that subsequent doses are unlikely to produce vomiting. Nausea and vomiting are significantly more common in ambulatory than in recumbent patients. Narcotic analgesics, including hydromorphone, increase the tone and decrease the propulsive contractions of the smooth muscle of the gastrointestinal tract. The resultant prolongation in gastrointestinal transit time is responsible for hydromorphone’s constipating effect. Because narcotics may increase biliary tract pressure, some patients with biliary colic may experience worsening rather than relief of pain.
While narcotics generally increase the tone of urinary tract smooth muscle, the net effect tends to be variable, in some cases producing urinary urgency, in others, difficulty in urination. Narcotics have also been reported to cause antidiuretic hormone (ADH) to be released, thereby reducing urine output.
In therapeutic dosage, opiates do not usually exert major effects on the cardiovascular system. However, some patients exhibit a propensity to develop orthostatic hypotension and fainting. Rapid intravenous injection is more likely to precipitate a fall in blood pressure than are intramuscular or subcutaneous injections.
Narcotic analgesics cause histamine release, which appears to be responsible for wheals or urticaria sometimes seen at the site of injection. Histamine release may also produce dilation of cutaneous blood vessels, with resultant flushing of the face and neck, pruritus and sweating.
Hydromorphone is well absorbed after parenteral administration.
After intramuscular administration, hydromorphone has a slightly more rapid onset and slightly shorter duration of analgesia than morphine. The major pathway of hydromorphone metabolism is conjugation with glucuronic acid in the liver; hydromorphone glucuronide is excreted primarily in the urine.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Hydromorphone Hydrochloride Injection is contraindicated in patients with a known hypersensitivity to hydromorphone, in the presence of an intracranial lesion associated with increased intracranial pressure and whenever ventilatory function is depressed (chronic obstructive pulmonary disease, cor pulmonale, emphysema, kyphoscoliosis, status asthmaticus). [See WARNINGS.]
Narcotic analgesics, including hydromorphone, are contraindicated in premature infants or during labor when delivery of a premature infant is anticipated.
-
WARNINGS
RESPIRATORY DEPRESSION
Hydromorphone produces dose-related respiratory depression by acting directly on brain stem respiratory centers. Hydromorphone also affects centers that control respiratory rhythm and may produce irregular and periodic breathing.
HEAD INJURY AND INCREASED INTRACRANIAL PRESSURE
The respiratory depressant effects of hydromorphone and its capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a pre-existing increase in intracranial pressure.
Furthermore, hydromorphone produces adverse reactions which may obscure the clinical course of patients with head injuries. In such patients, hydromorphone must be used with extreme caution and only if its use is deemed essential.
ACUTE ABDOMINAL CONDITIONS
The administration of hydromorphone may obscure the diagnosis or clinical course of patients with acute abdominal conditions.
ASTHMA AND OTHER RESPIRATORY CONDITIONS
Hydromorphone should be used with extreme caution in patients having an acute asthmatic attack, patients with chronic obstructive pulmonary disease or cor pulmonale, patients having a substantially decreased respiratory reserve and patients with pre-existing respiratory depression, hypoxia or hypercapnia. In such patients, even usual therapeutic doses of narcotics may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea.
INTRAVENOUS USE
If necessary, hydromorphone may be given intravenously, but the injection should be given very slowly (over a period of at least 3 to 5 minutes). Rapid intravenous injection of narcotic analgesics, including hydromorphone, increases the incidence of adverse reactions; severe respiratory depression, apnea, hypotension, peripheral circulatory collapse, cardiac arrest, as well as anaphylactoid reactions, have occurred. Hydromorphone should not be administered intravenously unless a narcotic antagonist and the facilities for resuscitation and assisted or controlled respiration are immediately available. When hydromorphone is given parenterally, especially intravenously, the patient should be lying down.
HYPOTENSIVE EFFECT
The administration of hydromorphone may result in severe hypotension in an individual whose ability to maintain blood pressure has already been compromised by a depleted blood volume or concurrent administration of drugs such as the phenothiazines or certain anesthetics.
Hydromorphone may produce orthostatic hypotension in ambulatory patients.
-
PRECAUTIONS
GENERAL
Narcotic analgesics, including hydromorphone, should be administered with caution and initial dose reduced in patients with acute abdominal conditions, convulsive disorders, significant hepatic or renal impairment, fever, hypothyroidism, Addison’s disease, ulcerative colitis, prostatic hypertrophy, urethral stricture, patients with recent gastrointestinal or urinary tract surgery and in elderly or debilitated patients. As with any narcotic analgesic agent, the possibility of respiratory depression should be kept in mind and the usual precautions observed.
Caution must be used when injecting any opioid subcutaneously or intramuscularly into chilled areas or in patients with hypotension or shock, since impaired perfusion may prevent complete absorption; if repeated injections are administered, an excessive amount may be suddenly absorbed if normal circulation is reestablished.
Hydromorphone suppresses the cough reflex; as with all narcotics, caution should be exercised when hydromorphone is used postoperatively and in patients with pulmonary disease.
INFORMATION FOR PATIENTS
Hydromorphone may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a vehicle or operating machinery. The concomitant use of alcohol or other central nervous system depressants, including sedatives, hypnotics, tranquilizers, phenothiazines and antihistamines may have an additive effect. Hydromorphone, like other narcotic analgesics, may produce orthostatic hypotension in ambulatory patients. Patients should be cautioned accordingly.
DRUG INTERACTIONS
Hydromorphone should be administered cautiously and in reduced dosage to avoid additive effects when other central nervous system depressants including other narcotic analgesics, general anesthetics, phenothiazines, tricyclic antidepressants, sedative-hypnotics or other CNS depressants (including alcohol) are given concomitantly.
Whenever concomitant therapy with MAO inhibitors and narcotic analgesics, including hydromorphone is to be used, an initial small test dose is advisable to allow observation of excessive narcotic effects or MAOI interaction.
DRUG/LABORATORY TEST INTERACTIONS
Because narcotic analgesics may increase biliary tract pressure, with resultant increase in plasma amylase or lipase levels, determination of these enzyme levels may be unreliable for 24 hours after a narcotic analgesic has been given.
CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
Long-term animal studies have not been performed to assess the carcinogenic potential of hydromorphone, nor are there any other animal or human data available concerning carcinogenesis, mutagenesis or impairment of fertility with this drug.
PREGNANCY
Teratogenic Effects - Pregnancy Category C.
Hydromorphone has been shown to be teratogenic in golden hamsters with a minimal effective teratogenic dose of 19 mg/kg, when given in doses 600 times the usual therapeutic dose in humans. There are no adequate and well-controlled studies in pregnant women. Hydromorphone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects.
Dependence has been reported in newborns whose mothers took opiates regularly during pregnancy. The withdrawal signs include irritability and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting and fever. Signs usually appear during the first few days of life.
LABOR AND DELIVERY
Hydromorphone crosses the placental barrier. The closer to delivery and the larger the dose used, the greater the possibility of respiratory depression in the newborn. Hydromorphone should be avoided during labor if delivery of a premature infant is anticipated. If the mother has received narcotic analgesics during labor, newborn infants should be observed closely for signs of respiratory depression. Resuscitation may be required (see OVERDOSAGE). The effect of hydromorphone, if any, on the later growth, development and functional maturation of the child is unknown.
Clinical studies have failed to demonstrate any effect of morphine on the uterus itself or on the pattern of contractions during labor. However, therapeutic doses of morphine have been reported to somewhat increase the duration of labor. The mechanism involved, the clinical significance of this observation, if any, or its relevance to other narcotic analgesics, including hydromorphone, are unknown.
NURSING MOTHERS
It is not known whether hydromorphone is excreted in human milk. However, detectable amounts of other narcotic analgesics have been found in human milk. Although these levels are not considered to be clinically significant after usual therapeutic dosage, since there is the potential for serious adverse reactions in nursing infants from hydromorphone, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
PEDIATRIC USE
Narcotic analgesics, including hydromorphone, should not be used in premature infants (see CONTRAINDICATIONS). Narcotics are reported to cross the immature blood-brain barrier to a greater extent, thereby producing disproportionate respiratory depression.
Safety and effectiveness of hydromorphone in infants and children have not been established and the optimal pediatric dose has not been determined.
-
ADVERSE REACTIONS
The major hazards of hydromorphone, as with other narcotic analgesics, are respiratory depression and, to a lesser degree, circulatory depression; respiratory arrest, shock and cardiac arrest have occurred, particularly with overdosage or rapid intravenous administration. Anaphylactoid reactions have been reported when phenanthrene alkaloids of opium are administered intravenously.
CENTRAL NERVOUS SYSTEM:
Sedation, drowsiness, mental clouding, headache, tremor, visual disturbances, weakness, agitation, lethargy, impairment of mental and physical performance, uncoordinated muscle movements, anxiety, fear, euphoria, dysphoria, dizziness, psychological dependence and mood changes.
GASTROINTESTINAL SYSTEM:
Nausea and vomiting occur more frequently in ambulatory than in recumbent patients. Hydromorphone may produce constipation, biliary tract spasm and can increase intraluminal pressure which may endanger surgical anastomosis. Patients with chronic ulcerative colitis may experience increased colonic motility; in patients with acute ulcerative colitis, toxic dilatation has been reported with narcotics.
CARDIOVASCULAR SYSTEM:
Circulatory depression, peripheral circulatory collapse and cardiac arrest have occurred after rapid intravenous injection. Orthostatic hypotension and fainting may occur, especially if a patient stands up suddenly after receiving an injection of hydromorphone. Additionally, hydromorphone may cause tachycardia, bradycardia and palpitations.
GENITOURINARY SYSTEM:
Oliguria, ureteral spasm, spasm of vesical sphincters and urinary retention have been reported.
RESPIRATORY DEPRESSION:
Hydromorphone produces dose-related respiratory depression by acting directly on brain stem respiratory centers. Hydromorphone also affects centers that control respiratory rhythm and may produce irregular and periodic breathing. If significant respiratory depression occurs, it may be antagonized by the use of naloxone hydrochloride. In patients who are physically dependent, small doses of naloxone may be sufficient not only to antagonize respiratory depression but also to precipitate withdrawal phenomena. The dose of naloxone should, therefore, be adjusted accordingly in such patients. Since the duration of action of hydromorphone may exceed that of the antagonist, the patient should be kept under continued surveillance; repeated doses of the antagonist may be required to maintain adequate respiration. Apply other supportive measures when indicated.
ALLERGIC:
Allergic reactions to opiates occur infrequently; pruritus, urticaria and other skin rashes are most common. Anaphylactoid reactions have been reported following intravenous administration of opiates.
OTHER:
Opiate-induced histamine release may be responsible for the flushing of the face, sweating and pruritus often seen with these drugs. Wheals and urticaria at the site of injection are probably related to histamine release. Local tissue irritation, pain and induration have been reported following repeated subcutaneous injection.
-
DRUG ABUSE AND DEPENDENCE
CONTROLLED SUBSTANCE
Hydromorphone Hydrochloride Injection is a Schedule II controlled narcotic substance.
ABUSE
Hydromorphone is known to be subject to abuse. Opiates produce relaxation, indifference to pain and stress, lethargy and euphoria. Patients who receive narcotics regularly for more than a few days may exhibit mild symptoms, which may not be recognized as withdrawal, upon discontinuation of therapy. However, the overwhelming majority of patients who receive opiates for medical reasons do not develop drug-seeking behavior or compulsive drug use. Personality characteristics play a major role in determining which patients are likely to abuse drugs. Hydromorphone must be administered only under close supervision to patients with a history of drug abuse or dependence.
DEPENDENCE
Psychological dependence, physical dependence and tolerance are known to occur with hydromorphone. Therefore, hydromorphone should be prescribed and administered with caution.
The severity of the abstinence syndrome is related to the degree of dependence, the abruptness of withdrawal and the drug used. If the abstinence syndrome is precipitated by administration of a narcotic antagonist, symptoms appear within a few minutes and are maximal within thirty minutes. Administration of a narcotic antagonist as a means of detecting dependence is not usually recommended. Withdrawal symptoms in patients dependent on hydromorphone include yawning; sweating; lacrimation; rhinorrhea; a restless, tossing sleep; dilated pupils; goose flesh; irritability; tremor; nausea; vomiting and diarrhea. Treatment of the abstinence syndrome is primarily symptomatic and supportive, including maintenance of proper fluid and electrolyte balance.
Tolerance, in which increasingly large doses are required in order to produce the same degree of analgesia, is manifested initially by a shortened duration of analgesic effect and subsequently by decreases in the intensity of analgesia. The rate of development of tolerance varies among patients.
-
OVERDOSAGE
SIGNS AND SYMPTOMS
Serious overdosage with hydromorphone is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin and sometimes bradycardia and hypotension. The triad of coma, pinpoint pupils and respiratory depression is strongly suggestive of opiate poisoning. In severe overdosage, particularly by the intravenous route, apnea, circulatory collapse, cardiac arrest and death may occur.
It is difficult to determine what constitutes a standard toxic or lethal dose. Infants and children are believed to be relatively more sensitive to opiates on a body-weight basis. Elderly patients are also comparatively intolerant to opiates.
TREATMENT
Primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and assisted or controlled ventilation. The narcotic antagonist naloxone hydrochloride is a specific antidote against respiratory depression which may result from overdosage or unusual sensitivity to narcotics, including hydromorphone. Therefore, an appropriate dose of naloxone hydrochloride should be administered, preferably by the intravenous route in conjunction with ventilatory assistance. Since the duration of action of hydromorphone may exceed that of the antagonist, the patient should be kept under continued surveillance; repeated doses of the antagonist may be required to maintain adequate respiration. An antagonist should not be administered in the absence of clinically significant respiratory or cardiovascular depression. Oxygen, intravenous fluids, vasopressors and other supportive measures should be employed as indicated.
The patient should be closely observed for a rise in temperature or pulmonary complications that may signal the need for institution of antibiotic therapy.
NOTE: In an individual physically dependent on narcotics, the administration of the usual dose of a narcotic antagonist will depend on the degree of physical dependence and the dose of antagonist administered. The use of narcotic antagonists in such individuals should be avoided if possible. If a narcotic antagonist must be used to treat serious respiratory depression in the physically dependent patient, the antagonist should be administered with extreme care and only one-tenth to one-fifth the usual initial dose administered.
-
DOSAGE AND ADMINISTRATION
The usual starting dose is 1-2 mg subcutaneously orintramuscularly every 4 to 6 hours as necessary for pain control. The dose should be adjusted according to severity of pain, as well as the patient’s underlying disease, age and size. Severe pain can usually be controlled by 3-4 mg every 4 to 6 hours as required. Patients with terminal cancer may become tolerant to narcotic analgesics and may, therefore, require higher doses for adequate pain relief. Should intravenous administration be necessary, the injection should be given very slowly (over at least 3 to 5 minutes, depending on the dose) [see WARNINGS, INTRAVENOUS USE]. A gradual increase in dosage may be required if analgesia is inadequate, tolerance occurs or if pain severity increases. The first sign of tolerance is usually a reduced duration of effect.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
-
HOW SUPPLIED
Hydromorphone Hydrochloride Injection, USP is available in the following:
2 mg/mL
20 mL Multiple Dose vials packaged individually (NDC 0641-2341-41)STORAGE
PROTECT FROM LIGHT: Keep covered in carton. Store at 20° to 25°C (68° to 77°F), excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Do not use the injection if it is more than slightly discolored or contains a precipitate.
To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689, or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For Product Inquiry call 1-877-845-0689.
Manufactured by:
Hikma Pharmaceuticals USA Inc.
Berkeley Heights, NJ 07922Revised April 2020
462-259-02
-
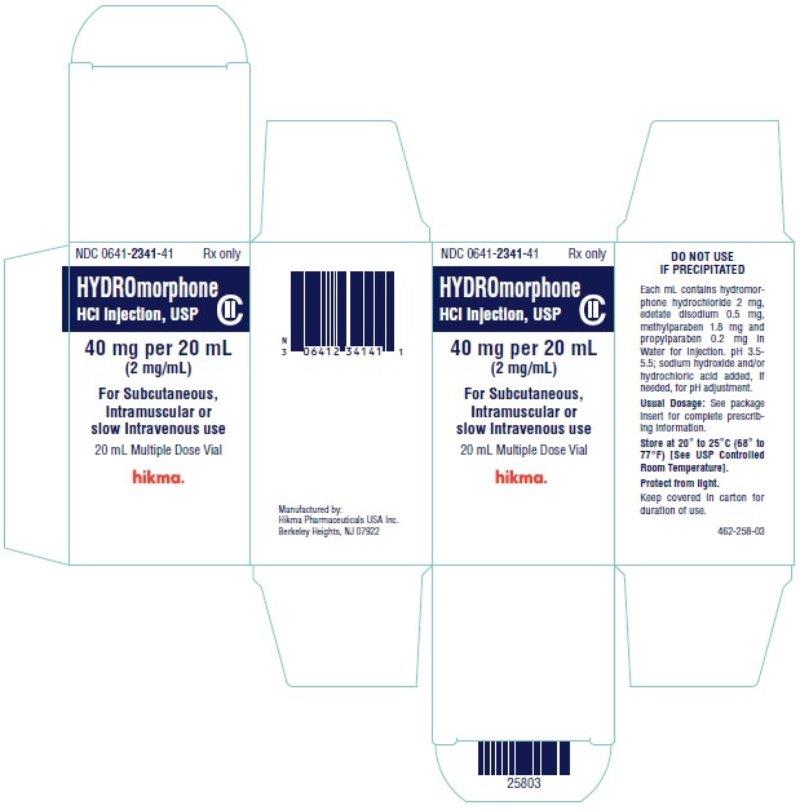
PRINCIPAL DISPLAY PANEL
NDC 0641-2341-39 Rx only
Hydromorphone
HCl Injection, USP CII
40 mg per 20 mL (2 mg/mL)
For Subcutaneous,
Intramuscular or slow
Intravenous use
20 mL Multiple Dose Vial

NDC 0641-2341-41 Rx only
Hydromorphone
HCl Injection, USP CII
40 mg per 20 mL
(2 mg/mL)
For Subcutaneous,
Intramuscular or
slow Intravenous use
20 mL Multiple Dose Vial
-
INGREDIENTS AND APPEARANCE
HYDROMORPHONE HYDROCHLORIDE
hydromorphone hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0641-0121 Route of Administration INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROMORPHONE HYDROCHLORIDE (UNII: L960UP2KRW) (HYDROMORPHONE - UNII:Q812464R06) HYDROMORPHONE HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) 0.5 mg in 1 mL METHYLPARABEN (UNII: A2I8C7HI9T) 1.8 mg in 1 mL PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.2 mg in 1 mL WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0641-0121-25 25 in 1 CARTON 01/01/1972 1 NDC:0641-0121-21 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/1972 HYDROMORPHONE HYDROCHLORIDE
hydromorphone hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0641-2341 Route of Administration INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROMORPHONE HYDROCHLORIDE (UNII: L960UP2KRW) (HYDROMORPHONE - UNII:Q812464R06) HYDROMORPHONE HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) 0.5 mg in 1 mL METHYLPARABEN (UNII: A2I8C7HI9T) 1.8 mg in 1 mL PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.2 mg in 1 mL WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0641-2341-41 20 in 1 CARTON 01/01/1972 1 NDC:0641-2341-39 20 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/1972 Labeler - Hikma Pharmaceuticals USA Inc. (946499746)