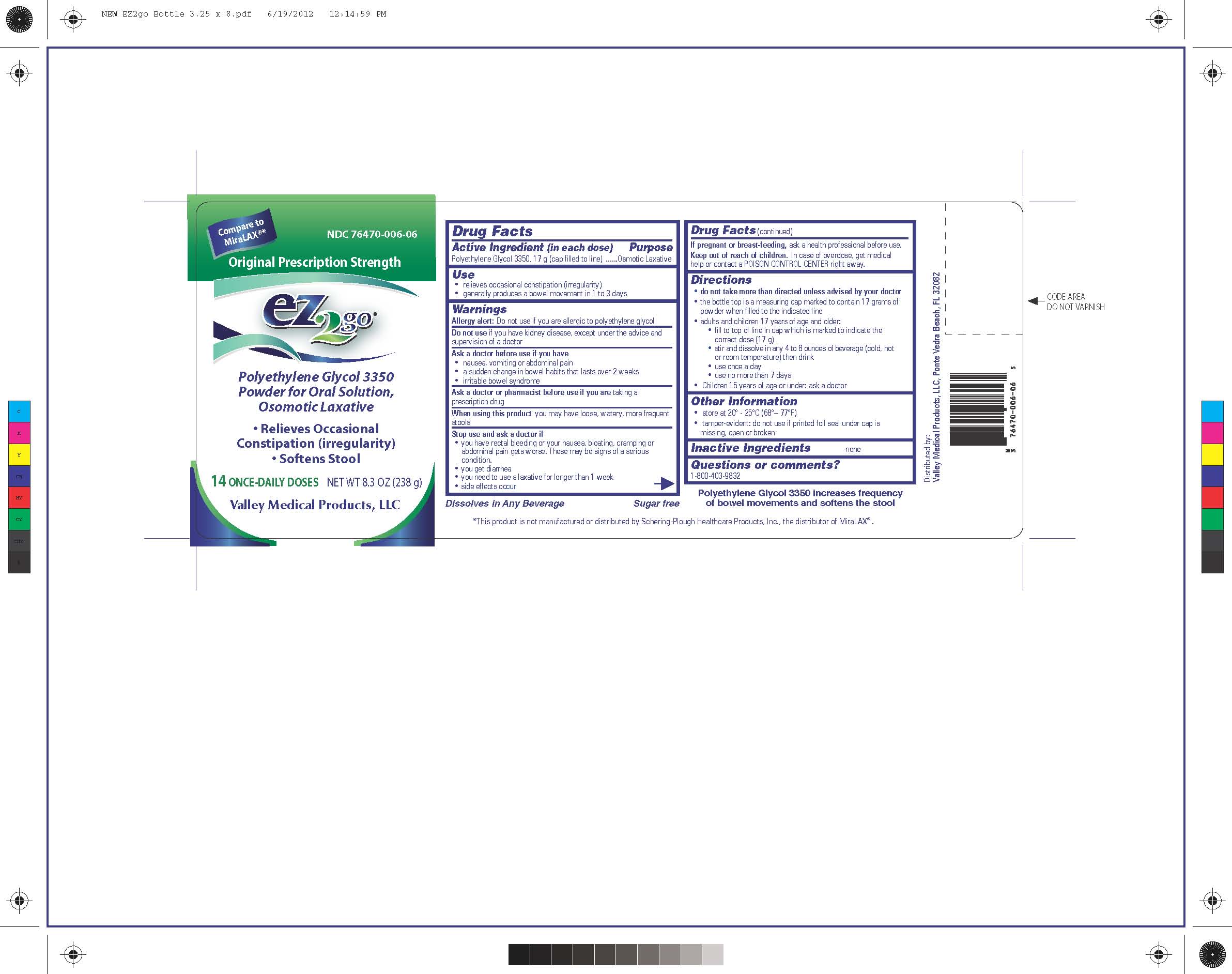
Label: EZ2GO PEG 3350- polyethylene glycol 3350 powder
-
Contains inactivated NDC Code(s)
NDC Code(s): 76470-006-06 - Packager: Valley Medical Products,LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 11, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INACTIVE INGREDIENT
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- ASK DOCTOR
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
-
DOSAGE & ADMINISTRATION
Directions
-do not take more than directed unless advised by your doctor
-the bottle top is a measuring cap marked to contain 17 grams of powder when filled to the indicated line
-adults and children 17 years of age and older:
-fill to top of line in cap which is marked to indicate the correct dose (17 g)
-stir and dissolve in any 4 to 8 ounces of beverage (cold, hot or room temperature) and then drink
-use once a day
-use no more than 7 days
-Children 16 years of age or under: ask a doctor
- QUESTIONS
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EZ2GO PEG 3350
polyethylene glycol 3350 powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76470-006 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 17 g in 17 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76470-006-06 238 g in 1 BOTTLE; Type 0: Not a Combination Product 10/20/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091077 10/20/2012 Labeler - Valley Medical Products,LLC (969389407)