Label: HELIUM gas
- NDC Code(s): 17575-005-01, 17575-005-02, 17575-005-03, 17575-005-04
- Packager: AGL Inhalation Therapy Co.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
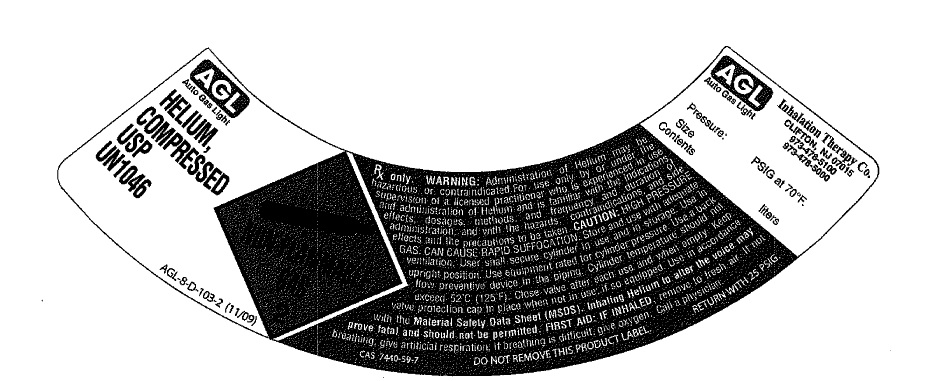
PRINCIPAL DISPLAY PANEL
AGL
Auto Gas Light
HELIUM,
COMPRESSED
USP
U.N. 1046
AGL-8-D-103-2 (11/09)
NON-FLAMMABLE GAS 2
Rx only. WARNING: Administration of helium may be hazardous or contraindicated. For use only by or under the supervision of a licensed practitioner who is experienced in the use of administration of helium and is familiar with indications, dosages and methods and frequency and duration of administration and with the hazards, contraindications and side effects and the precautions to be taken. CAUTION: HIGH PRESSURE GAS. CAN CAUSE RAPID SUFFOCATION.
Store and use with adequate ventilation. User shall secure cylinder in use and in storage. Use in upright position. Use equipment rated for cylinder pressure. Use a back flow preventive device in the piping. Cylinder temperature should not exceed 52 degrees C (125 degrees F). Close valve after each use and when empty. Keep valve protective cap in place when not in use, if so equipped. Use in accordance with the Material Safety Data Sheet (MSDS). Inhaling Helium to alter voice may prove fatal and should not be permitted. FIRST AID: IF INHALED, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Call a physician.
CAS 7440-59-7
DO NOT REMOVE THIS PRODUCT LABEL
RETURN WITH 25 PSIG.
AGL Inhalation Therapy Co. Clifton, NJ 07015
973-478-5100
973-478-5000
Pressure: PSIG at 70 degrees F.
Size
Contents liters
-
INGREDIENTS AND APPEARANCE
HELIUM
helium gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:17575-005 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HELIUM (UNII: 206GF3GB41) (HELIUM - UNII:206GF3GB41) HELIUM 990 mL in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17575-005-01 7986 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1966 2 NDC:17575-005-02 589 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1966 3 NDC:17575-005-03 352 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1966 4 NDC:17575-005-04 196 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1966 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205819 01/01/1966 Labeler - AGL Inhalation Therapy Co. (011186004) Establishment Name Address ID/FEI Business Operations AGL Inhalation Therapy Co. 011186004 manufacture(17575-005)