Label: BAL-CARE DHA- beta carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium carbonate, sodium feredetate, magnesium oxide, zinc oxide, cupric oxide, iron sucrose, iodine, omega 3 fatty acids kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 42546-180-30 - Packager: PruGen, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 1, 2012
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
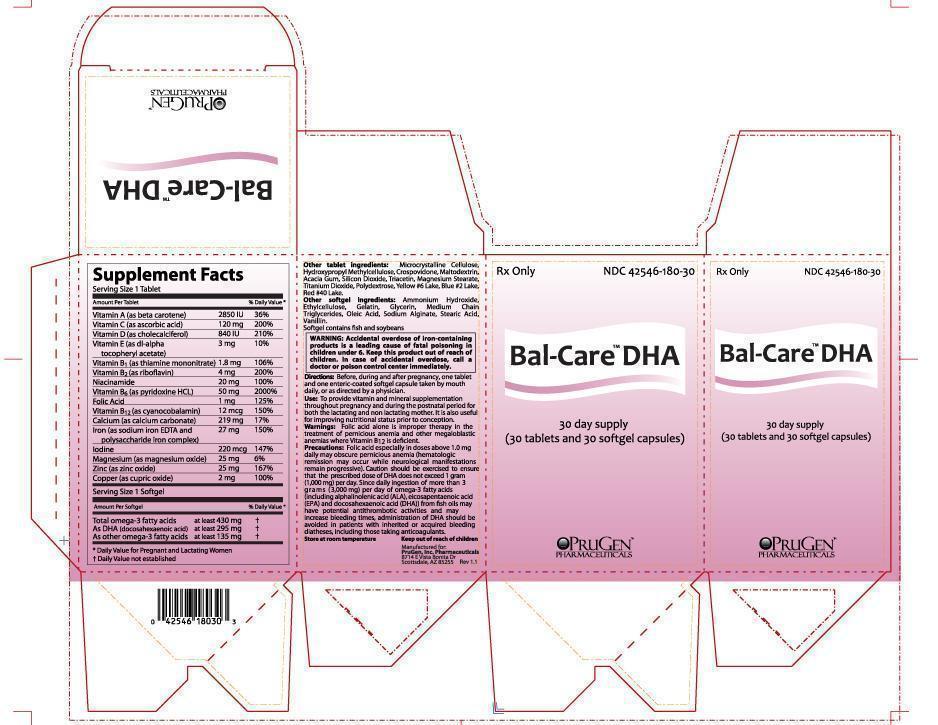
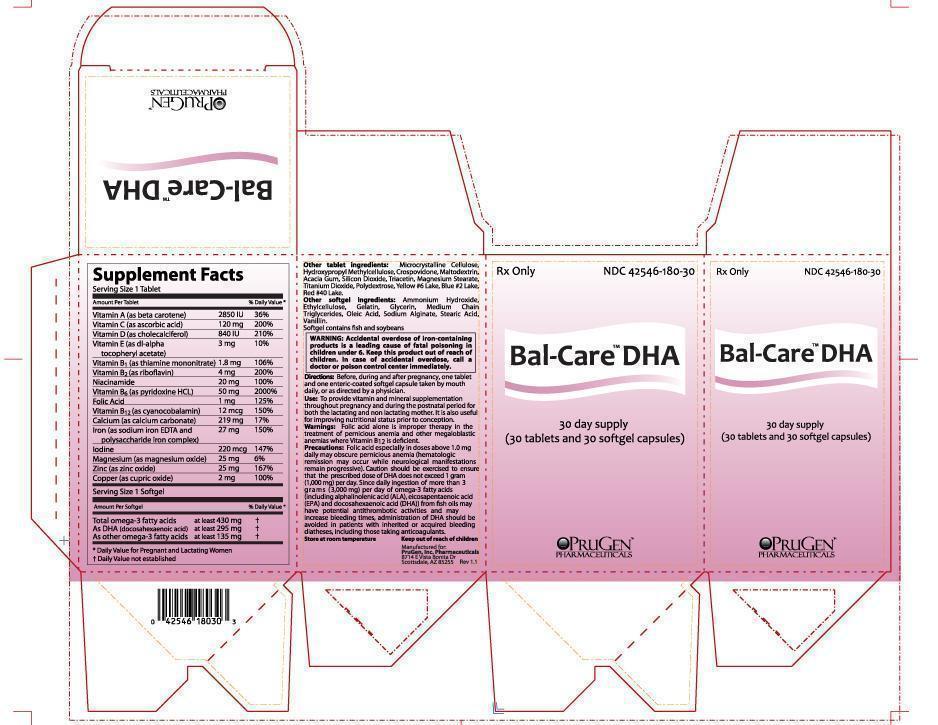
SUPPLEMENT FACTS
Serving Size 1 Tablet:
VITAMINS
A (beta carotene) . . . . . . . . . . . . . . . . . . . . . . . . . . 2,850 IU 36% Daily Value
C (ascorbic acid) . . . . . . . . . . . . . . . . . . . . . . . . . . 120 mg 200% Daily Value
D (cholecalciferol) . . . . . . . . . . . . . . . . . . . . . . . . .840 IU 210% Daily Value
E (dl-alpha tocopheryl acetate) . . . . . . . . . . . . . .3 mg 10% Daily Value
B1 (thiamine mononitrate) . . . . . . . . . . . . . . . . . . .1.8 mg 106% Daily Value
B2 (riboflavin) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4 mg 200% Daily Value
Niacinamide . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 mg 100% Daily Value
B6 (pyridoxine hydrochloride) . . . . . . . . . . . . . . .50 mg 2000% Daily Value
Folic acid . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1 mg 125% Daily Value
B12 (cyanocobalamin) . . . . . . . . . . . . . . . . . . . . . . 12 mcg 150% Daily Value
MINERALS
Calcium (calcium carbonate) . . . . . . . . . . . . . . . . 219 mg 17% Daily Value
Iron (as sodium iron EDTA and polysaccharide iron complex) . . .27 mg 150% Daily Value
Magnesium (magnesium oxide) . . . . . . . . . . . . . 25 mg 6% Daily Value
Zinc (zinc oxide) . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25 mg 167% Daily Value
Copper (cupric oxide) . . . . . . . . . . . . . . . . . . . . . . . .2 mg 100% Daily Value
Service Size 1 Softgel:
430 mg purified omega-3 fatty acids including:
At least 295 mg DHA (docosahexaenoic acid); also contains at least 135 mg of other omega-3 fatty acids.
OTHER TABLET INGREDIENTS:
Microcrystalline Cellulose, Hydroxypropyl Methylcellulose, Crospovidone, Maltodextrin, Acacia Gum, Silicon Dioxide, Triacetin, Magnesium Stearate, Titanium Dioxide, Polydextrose, Yellow #6 Lake, Blue #2 Lake, Red #40 Lake.
OTHER SOFTGEL INGREDIENTS:
Ammonium Hydroxide, Ethylcellulose, Gelatin, Glycerin, Medium Chain Triglycerides, Oleic Acid, Sodium Alginate, Stearic Acid, Vanillin.
Softgel contains fish and soybeans
-
INSTRUCTIONS FOR USE
Directions:
Before, during and after pregnancy, one tablet and one enteric-coated softgel capsule taken by mouth daily, or as directed by a physician.
Use: To provide vitamin and mineral supplementation throughout pregnancy and during the postnatal period for both the lactating and non lactating mother. It is also useful for improving nutritional status prior to conception.
-
WARNINGS AND PRECAUTIONS
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
Warnings: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient.
Precautions: Folic acid especially in doses above 1.0 mg daily may obscure pernicious anemia (hematologic remission may occur while neurological manifestations remain progressive). Caution should be exercised to ensure that the prescribed dose of DHA does not exceed 1 gram (1,000 mg) per day. Since daily ingestion of more than 3 grams (3,000 mg) per day of omega-3 fatty acids (including alphalinolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)) from fish oils may have potential antithrombotic activities and may increase bleeding times, administration of DHA should be avoided in patients with inherited or acquired bleeding diatheses, including those taking anticoagulants.
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BAL-CARE DHA
beta carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium carbonate, sodium feredetate, magnesium oxide, zinc oxide, cupric oxide, iron sucrose, iodine, omega 3 fatty acids kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42546-180 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42546-180-30 6 in 1 CARTON 1 1 in 1 BLISTER PACK Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 30 Part 2 30 Part 1 of 2 BAL-CARE DHA
beta carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium carbonate, sodium feredetate, magnesium oxide, zinc oxide, cupric oxide, iron sucrose, iodine, tabletProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BETA CAROTENE (UNII: 01YAE03M7J) (VITAMIN A - UNII:81G40H8B0T) BETA CAROTENE 2850 [iU] ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 120 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 840 [iU] .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL ACETATE, DL- - UNII:WR1WPI7EW8) .ALPHA.-TOCOPHEROL ACETATE, DL- 3 mg THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE - UNII:X66NSO3N35) THIAMINE MONONITRATE 1.8 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 4 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 20 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 50 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN .012 mg CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM - UNII:SY7Q814VUP) CALCIUM CARBONATE 219 mg SODIUM FEREDETATE (UNII: 403J23EMFA) (IRON - UNII:E1UOL152H7) SODIUM FEREDETATE 25.65 mg MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM - UNII:I38ZP9992A) MAGNESIUM OXIDE 25 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC - UNII:J41CSQ7QDS) ZINC OXIDE 25 mg CUPRIC OXIDE (UNII: V1XJQ704R4) (COPPER - UNII:789U1901C5) CUPRIC OXIDE 2 mg IRON SUCROSE (UNII: FZ7NYF5N8L) (IRON - UNII:E1UOL152H7) IRON SUCROSE 1.35 mg IODINE (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) IODINE .223 mg Product Characteristics Color pink Score no score Shape OVAL Size 19mm Flavor Imprint Code 102 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/01/2012 Part 2 of 2 BAL-CARE DHA
omega 3 fatty acids capsule, liquid filledProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OMEGA-3 FATTY ACIDS (UNII: 71M78END5S) (OMEGA-3 FATTY ACIDS - UNII:71M78END5S) OMEGA-3 FATTY ACIDS 430 mg Product Characteristics Color brown Score no score Shape OVAL Size 24mm Flavor Imprint Code none Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 05/01/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/01/2012 Labeler - PruGen, Inc. (929922750)