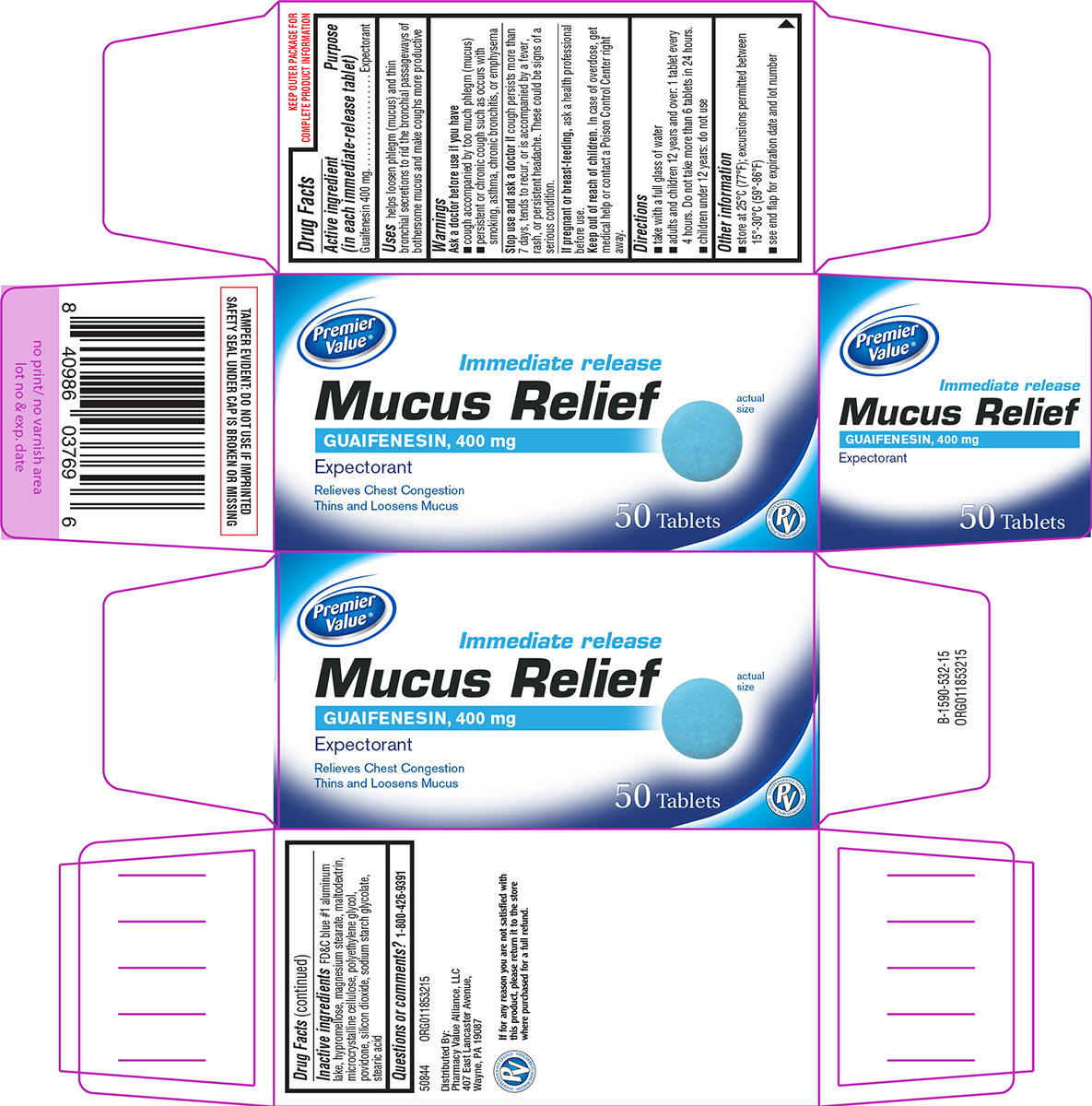
Label: MUCUS RELIEF- guaifenesin tablet, film coated
- NDC Code(s): 68016-545-50
- Packager: Chain Drug Consortium
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each immediate-release tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- cough accompanied by too much phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
Premier
Value®Immediate release
Mucus ReliefGUAIFENESIN, 400 mg
ExpectorantRelieves Chest Congestion
Thins and Loosens Mucusactual size
50 TabletsPV
INDEPENDENTLY TESTED
SATISFACTION GUARANTEEDTAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING50844 ORG011853215
Distributed By:
Pharmacy Value Alliance, LLC
407 East Lancaster Avenue,
Wayne, PA 19087If for any reason you are not satisfied with
this product, please return it to the store
where purchased for a full refund.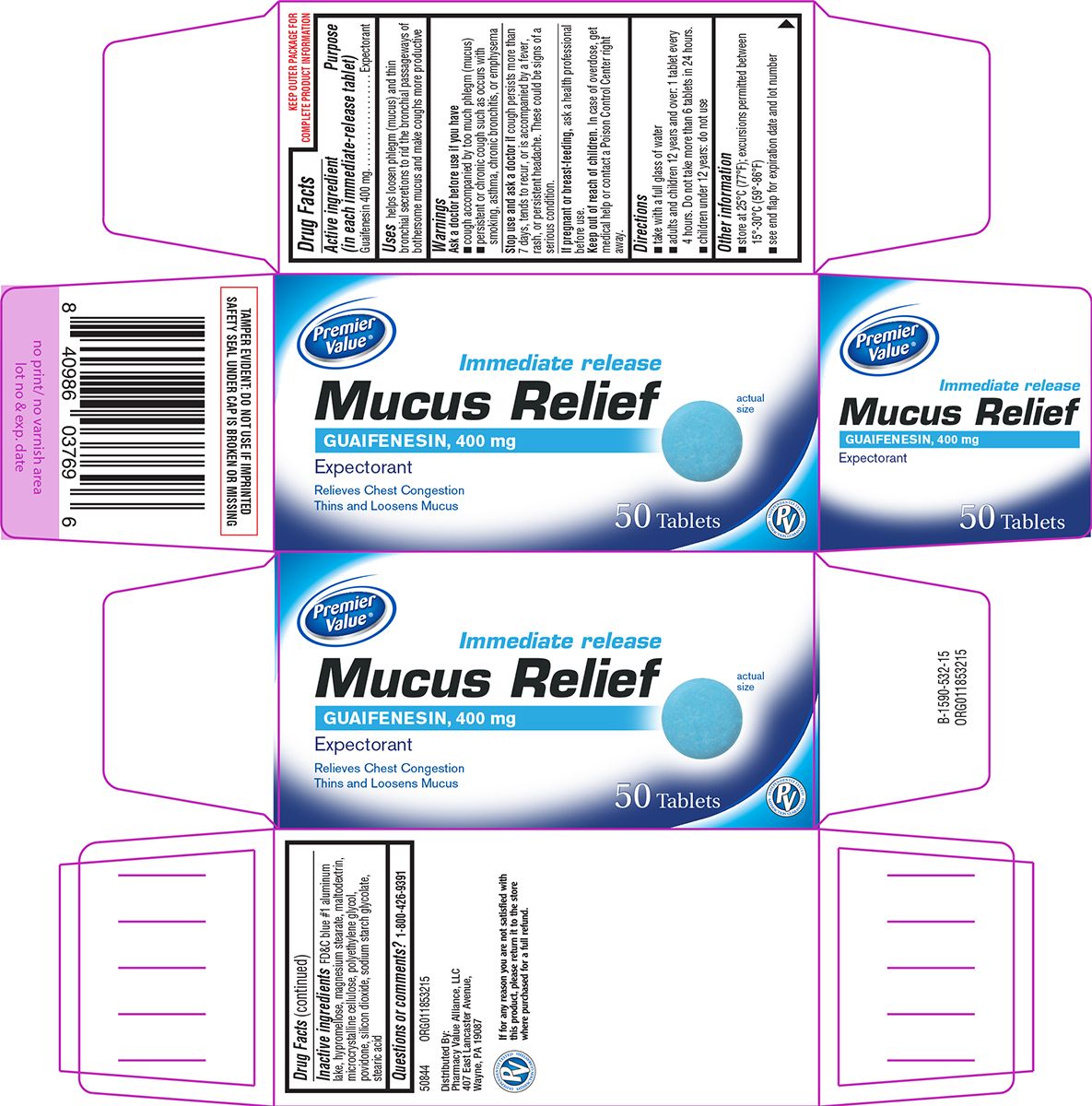
Premier Value 44-532
-
INGREDIENTS AND APPEARANCE
MUCUS RELIEF
guaifenesin tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68016-545 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 400 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color blue Score 2 pieces Shape ROUND Size 13mm Flavor Imprint Code 44;532 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68016-545-50 1 in 1 CARTON 01/09/2023 1 50 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/09/2023 Labeler - Chain Drug Consortium (101668460) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(68016-545) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(68016-545) , pack(68016-545) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(68016-545) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(68016-545) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(68016-545)