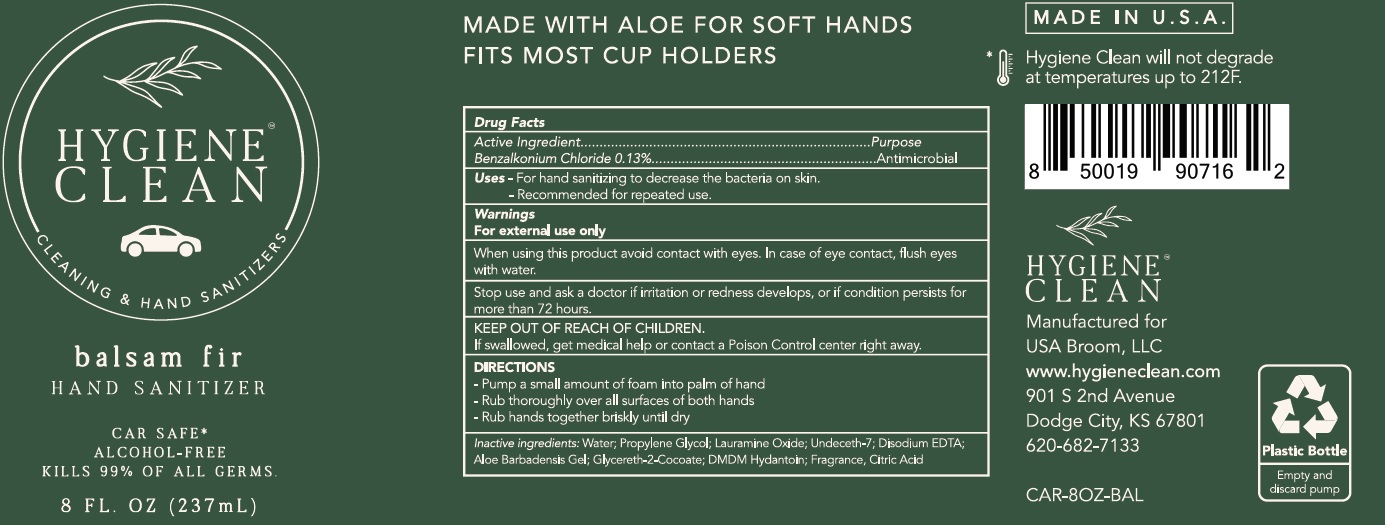
Label: HYGIENE CLEAN BALSAM FIR HAND SANITIZER ALCOHOL FREE- benzalkonium chloride liquid
- NDC Code(s): 80499-004-01, 80499-004-02
- Packager: USA Broom LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Uses
- Warnings
- DIRECTIONS
- Inactive ingredients:
- Package Labeling:(237 ml)
- Package Labeling:(237 ml)- 24 bottles
-
INGREDIENTS AND APPEARANCE
HYGIENE CLEAN BALSAM FIR HAND SANITIZER ALCOHOL FREE
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80499-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) LAURAMINE OXIDE (UNII: 4F6FC4MI8W) UNDECETH-7 (UNII: R6B5PCO2JN) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) GLYCERETH-2 COCOATE (UNII: JWM00VS7HC) DMDM HYDANTOIN (UNII: BYR0546TOW) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80499-004-01 237 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/30/2020 2 NDC:80499-004-02 24 in 1 BOX 09/30/2020 2 237 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 09/30/2020 Labeler - USA Broom LLC (117638854)