Label: FALLBACK SOLO- levonorgestrel tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 57297-853-11, 57297-853-13 - Packager: LUPIN LIMITED
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 4, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Indications
-
Warnings
Allergy alert: Do not use if you have ever had an allergic reaction to levonorgestrel.
Sexually transmitted diseases (STDs) alert: This product does not protect against HIV/AIDS or other STDs
Do not use
- if you are already pregnant (because it will not work)
- for regular birth control
When using this product you may have
- menstrual changes
- nausea
- lower stomach (abdominal) pain
- tiredness
- headache
- dizziness
- breast pain
- vomiting
- KEEP OUT OF REACH OF CHILDREN
-
SPL UNCLASSIFIED SECTION
■ women 17 years of age and older:
- take tablet as soon as possible within 72 hours (3 days) after unprotected sex. The sooner you take it the better it will work.
- if you vomit within 2 hours after taking the medication, call a healthcare professional to find out if you should repeat the dose.
- read the instructions, warnings and enclosed product leaflet before use .
- this product works mainly by preventing ovulation (egg release). It may also prevent fertilization of a released egg (joining of sperm and egg) or attachment of a fertilized egg to the uterus (implantation).
- tablet is enclosed in wallet seal. Do not use if the wallet seal is broken .
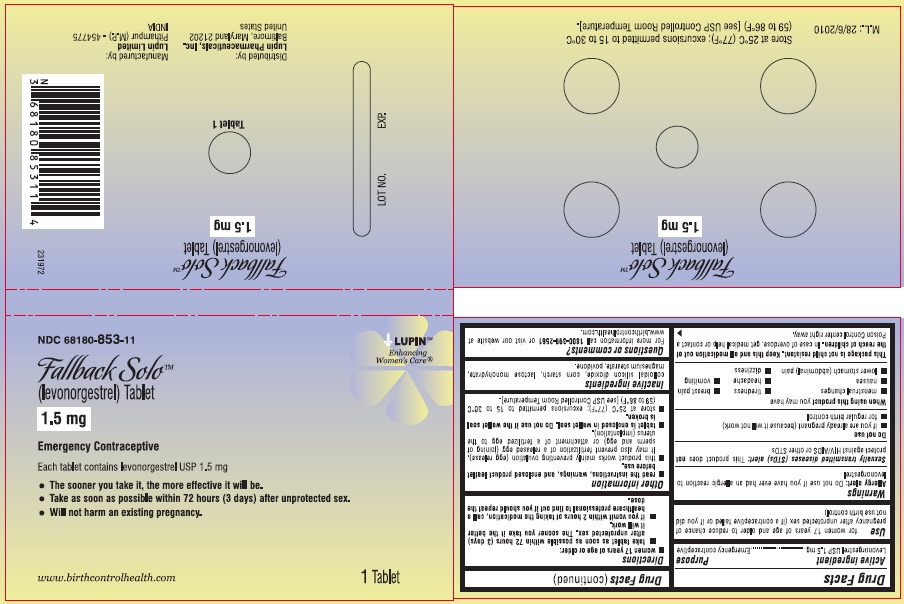
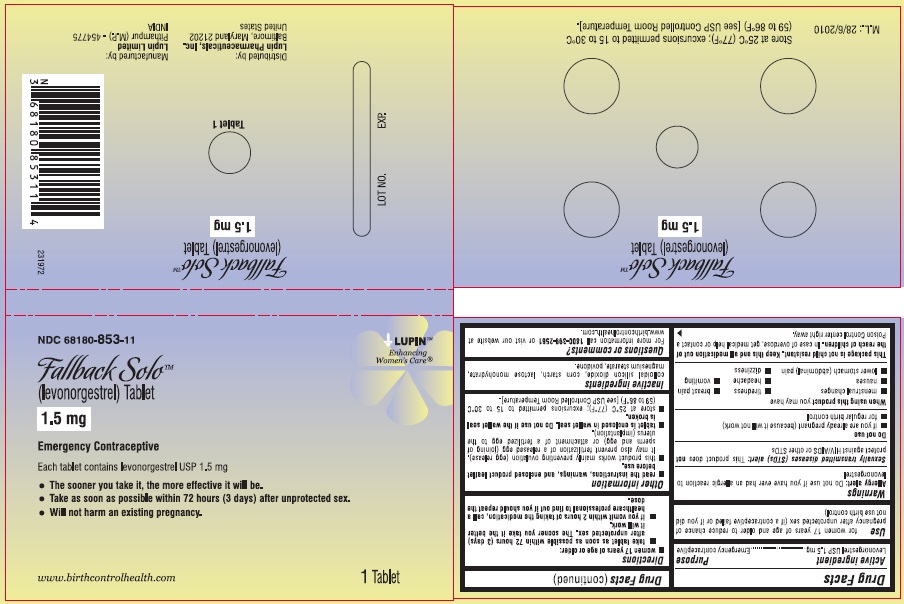
- store at 25°C (77°C); excursions permitted to 15 to 30°C (59 to 86°C) [see USP Controlled Room temperature].
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
-
SPL UNCLASSIFIED SECTION
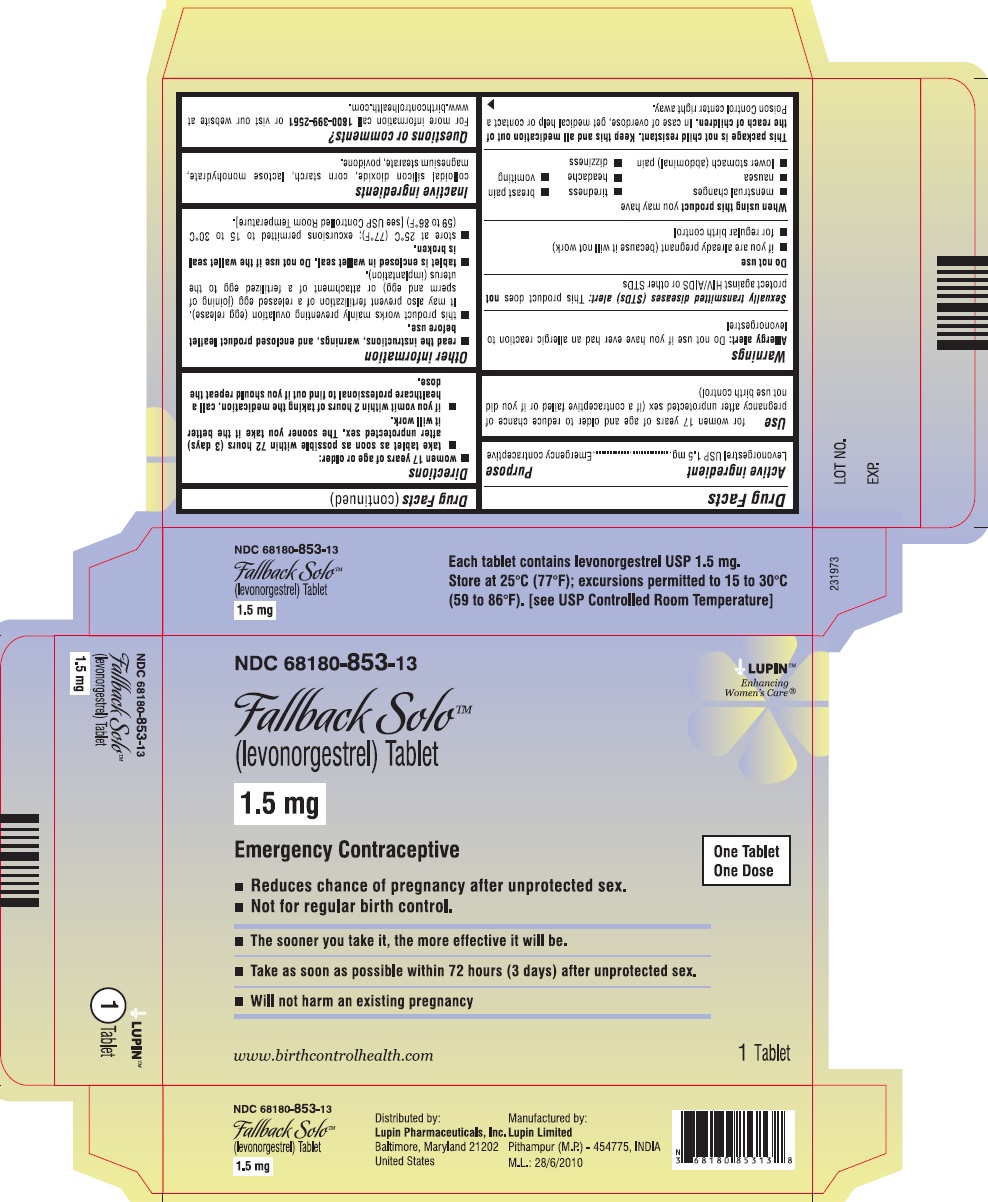
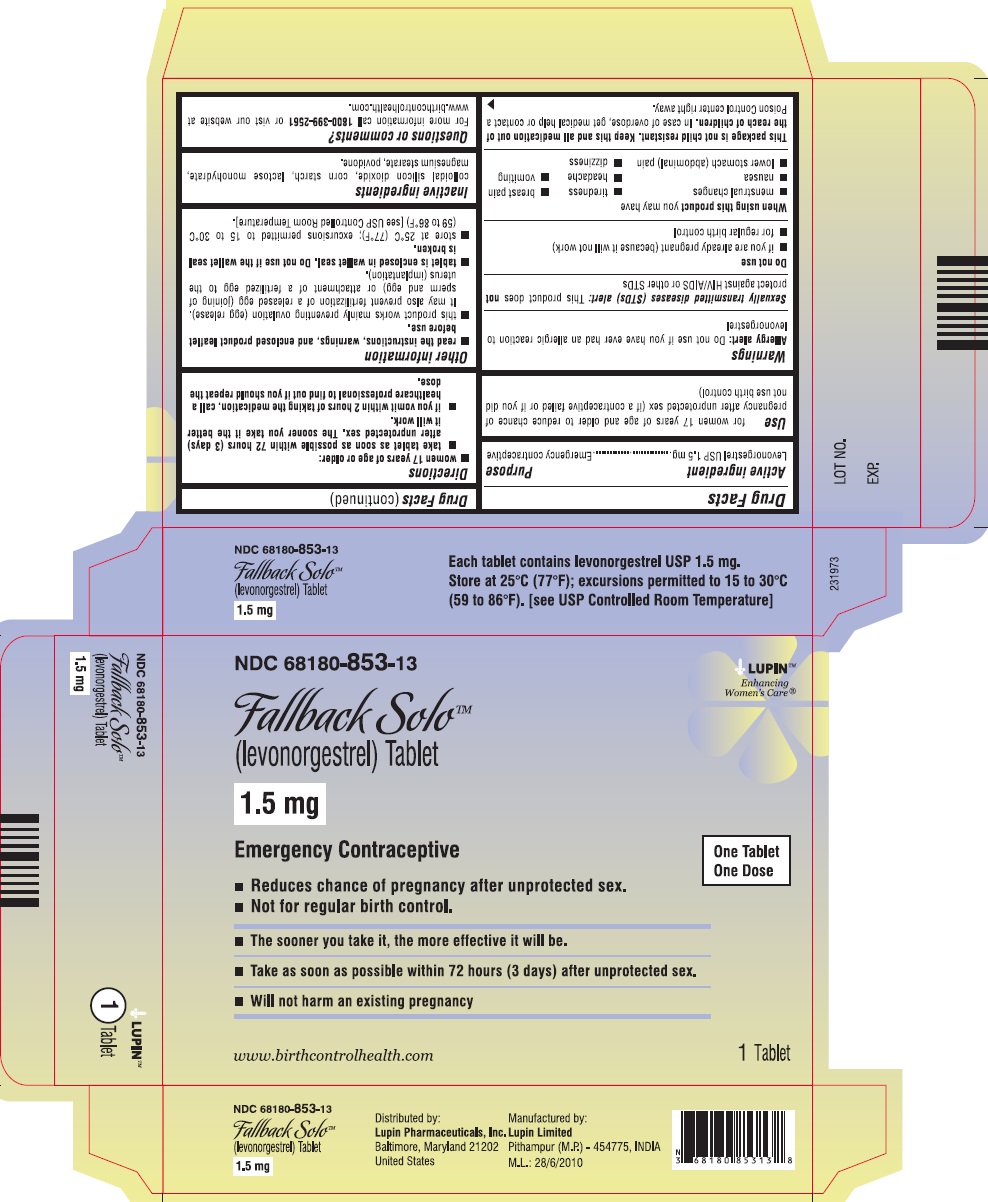
(levonorgestrel) Tablet, 1.5 mg
Emergency Contraceptive
One Tablet. One Dose.
What is Fallback Solo™?
Fallback Solo™ is emergency contraception that helps prevent pregnancy after birth control failure or unprotected sex. It is a backup method of preventing pregnancy and should not be used as regular birth control.
What Fallback Solo is not.
Fallback Solo will not work if you are already pregnant and will not affect an existing pregnancy. Fallback Solo will not protect you from HIV infection (the virus that causes AIDS) and other sexually transmitted diseases (STDs).
-
When should I use Fallback Solo?
The sooner you take emergency contraception, the better it works. You should use Fallback Solo within 72 hours (3 days) after you have had unprotected sex.
Fallback Solo is a backup or emergency method of birth control you can use when:
- your regular birth control was used incorrectly or failed
- you did not use any birth control method
-
SPL UNCLASSIFIED SECTION
When not to use Fallback Solo?
Fallback Solo should not be used:
- as a regular birth control method, because it's not as effective as regular birth control.
- if you are already pregnant, because it will not work.
- if you are allergic to levonorgestrel or any other ingredients in Fallback Solo.
Fallback Solo is one tablet with levonorgestrel, a hormone that has been used in many birth control pills for several decades. Fallback Solo contains a higher dose of levonorgestrel than birth control pills, but works in a similar way to prevent pregnancy. It works mainly by stopping the release of an egg from the ovary. It is possible that Fallback Solo may also work by preventing fertilization of an egg (the uniting of sperm with the egg) or by preventing attachment (implantation) to the uterus (womb).
How can I get the best results from Fallback Solo?
You have 72 hours (3 days) to try to prevent pregnancy after birth control failure or unprotected sex. The sooner you take Fallback Solo, the better it works.
How effective is Fallback Solo?
If Fallback Solo is taken as directed, it can significantly decrease the chance that you will get pregnant. About 7 out of every 8 women who would have gotten pregnant will not become pregnant.
How will I know Fallback Solo worked?
You will know Fallback Solo has been effective when you get your next period, which should come at the expected time, or within a week of the expected time. If your period is delayed beyond 1 week, it is possible you may be pregnant. You should get a pregnancy test and follow up with your healthcare professional.
Will I experience any side effects?
- some women may have changes in their period, such as a period that is heavier or lighter or a period that is early or late. If your period is more than a week late, you may be pregnant.
- If you have severe abdominal pain, you may have an ectopic pregnancy, and should get immediate medical attention.
- when used as directed, Fallback Solo is safe and effective. Side effects may include changes in your period, nausea, lower stomach (abdominal) pain, tiredness, headache, dizziness, and breast tenderness.
- if you vomit within 2 hours of taking the medication, call a healthcare professional to find out if you should repeat the dose.
What if I still have questions about Fallback Solo?
If you have questions or need more information, call our toll-free number, 1800-399-2561 or visit our website at www.birthcontrolhealth.com.
Other Information
Keep this and all medication out of reach of children:
In case of overdose, get medical help or contact a Poison Control Center right away at 1-800-222-1222.
Do not use if the wallet seal is opened.
Store at 25°C (77°F); excursions permitted to 15 to 30°C (59 to 86°F). [see USP Controlled Room Temperature].
Active Ingredient: levonorgestrel 1.5 mg
Inactive Ingredients: colloidal silicon dioxide, corn starch, lactose monohydrate, magnesium stearate, and povidone.
Fallback Solo™ is a trademark of Lupin Pharmaceuticals, Inc.
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland 21202
United States
Manufactured by:
Lupin Limited
Pithampur (M.P.) - 454 775
INDIA
June 2014 ID#: 231975
If you are sexually active, you should see a healthcare provider for routine checkups. Your healthcare provider will talk to you about and, if necessary, test you for sexually transmitted diseases, teach you about effective methods of routine birth control, and answer any other questions you may have.
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FALLBACK SOLO
levonorgestrel tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57297-853 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVONORGESTREL (UNII: 5W7SIA7YZW) (LEVONORGESTREL - UNII:5W7SIA7YZW) LEVONORGESTREL 1.5 mg Inactive Ingredients Ingredient Name Strength COLLOIDAL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE K30 (UNII: U725QWY32X) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE (white to off white) Score no score Shape ROUND (round biconvex) Size 9mm Flavor Imprint Code LU;S25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57297-853-13 1 in 1 CARTON 1 NDC:57297-853-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA201446 07/22/2014 Labeler - LUPIN LIMITED (675923163) Registrant - LUPIN LIMITED (675923163) Establishment Name Address ID/FEI Business Operations LUPIN LIMITED 650582310 manufacture(57297-853) , pack(57297-853)