Label: CERIANNA- fluoroestradiol f 18 injection
- NDC Code(s): 72874-001-01
- Packager: GE Healthcare Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 1, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CERIANNA™ safely and effectively. See full prescribing information for CERIANNA.
CERIANNA™ (fluoroestradiol F 18) Injection, for intravenous use
Initial U.S. Approval: 2020INDICATIONS AND USAGE
CERIANNA is a radioactive diagnostic agent indicated for use with positron emission tomography (PET) imaging for the detection of estrogen receptor (ER)-positive lesions as an adjunct to biopsy in patients with recurrent or metastatic breast cancer. (1)
Limitations of Use
Tissue biopsy should be used to confirm recurrence of breast cancer and to verify ER status by pathology. CERIANNA is not useful for imaging other receptors, such as human epidermal growth factor receptor 2 (HER2) and the progesterone receptor (PR). (1, 5.1)
DOSAGE AND ADMINISTRATION
- Recommended dose is 222 MBq (6 mCi), with a range of 111 MBq to 222 MBq (3 mCi to 6 mCi), administered as an intravenous injection over 1 to 2 minutes. (2.2)
- Recommended imaging start time is 80 minutes (range 20 minutes to 80 minutes) after drug administration. (2.4)
- See full prescribing information for additional preparation, administration, imaging, and radiation dosimetry information. (2)
DOSAGE FORMS AND STRENGTHS
Injection: 148 MBq/mL to 3,700 MBq/mL (4 mCi/mL to 100 mCi/mL) of fluoroestradiol F 18 in a multiple-dose vial. (3)
CONTRAINDICATIONS
- None. (4)
WARNINGS AND PRECAUTIONS
- Risk of Misdiagnosis. Do not use CERIANNA in lieu of biopsy when biopsy is indicated in patients with recurrent or metastatic breast cancer. Pathology or clinical characteristics that suggest a patient may benefit from systemic hormone therapy should take precedence over a discordant negative CERIANNA scan. (5.1)
- Radiation Risks. Ensure safe drug handling and patient preparation procedures to protect patients and health care providers from unintentional radiation exposure. (2.1, 2.3, 5.2)
ADVERSE REACTIONS
Reported adverse reactions include: injection-site pain and dysgeusia (6)
To report SUSPECTED ADVERSE REACTIONS, contact GE HealthCare at 1-800-654-0118 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Lactation: Interrupt breastfeeding. Advise a lactating woman to avoid breastfeeding for 4 hours after CERIANNA administration. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety - Drug Handling
2.2 Recommended Dosage and Administration Instructions
2.3 Patient Preparation
2.4 Image Acquisition
2.5 Image Interpretation
2.6 Radiation Dosimetry
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Misdiagnosis
5.2 Radiation Risks
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
11.1 Chemical Characteristics
11.2 Physical Characteristics
11.3 External Radiation
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety - Drug Handling
CERIANNA is a radioactive drug. Only authorized persons qualified by training and experience should receive, use, and administer CERIANNA. Handle CERIANNA with appropriate safety measures to minimize radiation exposure during administration [see Warnings and Precautions (5.2)]. Use waterproof gloves and effective radiation shielding, including syringe shields, when preparing and handling CERIANNA.
2.2 Recommended Dosage and Administration Instructions
Recommended Dosage
The recommended amount of radioactivity to be administered for PET imaging is 222 MBq (6 mCi), with a range of 111 MBq to 222 MBq (3 mCi to 6 mCi), administered as a single intravenous injection of 10 mL or less over 1 to 2 minutes.
Preparation and Administration
- For patient preparation instructions, see Dosage and Administration 2.3.
- Use aseptic technique and radiation shielding when withdrawing and administering CERIANNA.
- Visually inspect the radiopharmaceutical solution. Do not use if it contains particulate matter or if it is cloudy or discolored (CERIANNA is a clear, colorless solution).
- CERIANNA may be diluted with 0.9% Sodium Chloride Injection, USP.
- Assay the dose in a suitable dose calibrator prior to administration.
2.3 Patient Preparation
Assessment for Drug Interactions
Before administering CERIANNA, discontinue drugs that bind to ER (e.g., selective estrogen receptor modulators (SERMs) and selective estrogen receptor down-regulators (SERDs)) [see Drug Interactions (7)].
2.4 Image Acquisition
Position the patient supine with arms above the head, if possible. The recommended start time for image acquisition is 80 minutes after the intravenous administration of CERIANNA. Scan duration adapted from the range of 20 minutes to 30 minutes and imaging start times adapted within the range of 20 minutes to 80 minutes may be customized according to the equipment used and patient and tumor characteristics for optimal image quality.
2.5 Image Interpretation
Uptake of fluoroestradiol F 18 depends on ER density and function in tumors and physiologic tissue, including in liver, ovary, and uterus. Detection of ER-positive tumors should be based on comparison with tissue background outside of organs with high physiologic uptake and regions with high activity due to hepatobiliary and urinary excretion.
2.6 Radiation Dosimetry
Radiation absorbed dose estimates are shown in Table 1 for organs and tissues of adults from intravenous administration of CERIANNA. The radiation effective dose resulting from administration of 222 MBq (6 mCi) of CERIANNA to an adult weighing 70 kg is estimated to be 4.9 mSv. Critical organs include the liver, gallbladder, and uterus. When PET/CT is performed, exposure to radiation will increase by an amount dependent on the settings used for the CT acquisition.
Table 1. Estimated Radiation Absorbed Doses in Various Organs/Tissues in Adults Who Received FLUOROESTRADIOL F 18 Organ Mean Absorbed Dose Per Unit of Activity Administered (mGy/MBq) Adrenals 0.023 Brain 0.01 Breasts 0.009 Gallbladder 0.102 Lower large intestine 0.012 Small intestine 0.027 Stomach 0.014 Upper large intestine 0.03 Heart wall 0.026 Kidney 0.035 Liver 0.126 Lungs 0.017 Muscle 0.021 Ovaries 0.018 Pancreas 0.023 Red Marrow 0.013 Bone surface 0.014 Skin 0.005 Spleen 0.015 Testes 0.012 Thymus 0.014 Thyroid 0.012 Urinary bladder 0.05 Uterus 0.039 Lens 0.009 Effective dose = 0.022 mSv/MBq - 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Misdiagnosis
Inadequate Tumor Characterization and Other ER-Positive Pathology
Breast cancer may be heterogeneous within patients and across time. CERIANNA images ER and is not useful for imaging other receptors such as HER2 and PR. The uptake of fluoroestradiol F 18 is not specific for breast cancer and may occur in a variety of ER-positive tumors that arise outside of the breast, including from the uterus and ovaries. Do not use CERIANNA in lieu of biopsy when biopsy is indicated in patients with recurrent or metastatic breast cancer.
False Negative CERIANNA Scan
A negative CERIANNA scan does not rule out ER-positive breast cancer [see Clinical Studies (14)]. Pathology or clinical characteristics that suggest a patient may benefit from systemic hormone therapy should take precedence over a discordant negative CERIANNA scan.
5.2 Radiation Risks
Diagnostic radiopharmaceuticals, including CERIANNA, expose patients to radiation [see Dosage and Administration (2.6)]. Radiation exposure is associated with a dose-dependent increased risk of cancer. Ensure safe drug handling and patient preparation procedures to protect patients and health care providers from unintentional radiation exposure [see Dosage and Administration (2.1) and (2.3)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of CERIANNA was evaluated from published clinical studies of 1,207 patients with breast cancer receiving at least one fluoroestradiol F 18 administration. The following adverse reactions occurred at a rate < 1%:
- General disorders: injection-site pain
- Neurological and gastrointestinal disorders: dysgeusia
-
7 DRUG INTERACTIONS
Drugs that bind to the estrogen receptor (ER) may compete with the binding of fluoroestradiol F 18 and may reduce the detection of ER-positive lesions with CERIANNA.
Before administering CERIANNA, discontinue drugs that bind to the ER, such as SERMs and SERDs, for at least 5 biological half-lives (e.g., elacestrant for 11 days, tamoxifen for 8 weeks, and fulvestrant for 28 weeks) [see Dosage and Administration (2.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All radiopharmaceuticals, including CERIANNA, have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of radiation dose. Advise a pregnant woman of the potential risks of fetal exposure to radiation from administration of CERIANNA.
There are no available data on CERIANNA use in pregnant women. No animal reproduction studies using fluoroestradiol F 18 have been conducted to evaluate its effect on female reproduction and embryo-fetal development.
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of fluoroestradiol F 18 in human milk, or its effects on the breastfed infant or milk production. Lactation studies have not been conducted in animals. Advise a lactating woman to avoid breastfeeding for 4 hours after CERIANNA administration in order to minimize radiation exposure to a breastfed infant.
-
11 DESCRIPTION
11.1 Chemical Characteristics
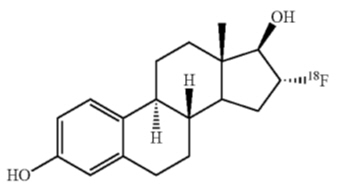
CERIANNA contains fluoroestradiol fluorine 18 (F 18), a synthetic estrogen analog. Chemically, fluoroestradiol F 18 is [18F]16α-fluoro-3,17β-diol-estratriene-1,3,5(10). The molecular weight is 289.37, and the structural formula is:
CERIANNA is a sterile, clear, colorless solution for intravenous injection, with an osmolarity of 340 mOsm. Its pH ranges between 5.5 to 8.0. The composition of the final product in 40 mL solution is fluoroestradiol no more than 5 mcg, fluoroestradiol F 18 148 MBq/mL to 3,700 MBq/mL (4 mCi/mL to 100 mCi/mL), sodium ascorbate 0.44% w/v in sodium chloride 0.9% w/v, and ethanol no more than 3.2% w/v.
11.2 Physical Characteristics
CERIANNA is radiolabeled with F 18, a cyclotron produced radionuclide that decays by positron emission to stable oxygen 18 with a half-life of 109.8 minutes. The principal photons useful for diagnostic imaging are the coincident pair of 511 keV gamma photons, resulting from the interaction of the emitted positron with an electron (Table 2).
Table 2. Principal Radiation Produced From Decay of Fluorine 18 Radiation Radiation Energy Level (keV) % Abundance Positron 249.8 96.9 Gamma 511 193.5 11.3 External Radiation
The point source air-kerma coefficient for F 18 is 3.75 × 10-17 Gy m2 / (Bq s). The first half-value thickness of lead (Pb) for F 18 gamma rays is approximately 6 mm. The relative reduction of radiation emitted by F 18 that results from various thicknesses of lead shielding is shown in Table 3. The use of 8 cm Pb decreases the radiation transmission (i.e., exposure) by a factor of about 10,000.
Table 3. Radiation Attenuation of 511 keV Gamma Rays by Lead Shielding Shield Thickness cm of Lead (Pb) Coefficient of Attenuation 0.6 0.5 2 0.1 4 0.01 6 0.001 8 0.0001 -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fluoroestradiol F 18 binds ER. The following binding affinity: Kd = 0.13 ± 0.02 nM, Bmax = 1901 ± 89 fmol/mg, and IC50 = 0.085 nM, was determined in an ER-positive human breast cancer cell line (MCF-7).
12.2 Pharmacodynamics
The relationship between fluoroestradiol F18 plasma concentrations and image interpretation has not been studied. Fluoroestradiol F18 uptake measured by PET in human tumors is directly proportional to tumor ER expression measured by in vitro assays.
12.3 Pharmacokinetics
Distribution
After intravenous injection, 95% of fluoroestradiol F 18 is bound to plasma proteins. Fluoroestradiol F 18 distributes primarily to hepatobiliary system, and also to small and large intestines, heart wall, blood, kidney, uterus and bladder.
Metabolism
Fluoroestradiol F 18 is metabolized in the liver. At 20 minutes after injection, approximately 20% of circulating radioactivity in the plasma is in the form of non-metabolized fluoroestradiol F 18. At 2 hours after injection, circulating fluoroestradiol F 18 levels are less than 5% of peak concentration.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No long-term studies in animals were performed to evaluate the carcinogenic potential of CERIANNA.
Mutagenesis
Fluoroestradiol was evaluated by in vitro bacterial reverse mutation assay (Ames test) and in vitro L5178Y/TK+/- mouse lymphoma mutagenesis assay. Fluoroestradiol was negative for genotoxicity by Ames test at up to 1.25 µg per plate for 5 tester strains (Salmonella typhimurium tester strains TA98, TA100, TA1535 and TA1537 and Escherichia Coli tester strain WP2 uvrA) in the presence or absence of S9 metabolic activation. Fluoroestradiol was negative for genotoxicity by L5178Y/TK+/- mouse lymphoma mutagenesis assay at up to 8 ng/mL in the absence or presence of S9 metabolic activation.
Potential in vivo genotoxicity of fluoroestradiol was evaluated in a rat micronucleus assay. In this assay, fluoroestradiol did not increase the number of micronucleated polychromatic erythrocytes (MN-PCEs) at 51 µg/kg/day, when given for 14 consecutive days. However, CERIANNA has the potential to be mutagenic because of the F 18 radioisotope.
-
14 CLINICAL STUDIES
The effectiveness of CERIANNA for detecting ER-positive non-primary breast cancer lesions was evaluated based on published study reports of fluoroestradiol F 18. Study 1 (NCT01986569) enrolled 90 women (median age 55 years, 39% premenopausal) with histologically confirmed invasive breast cancer. The patients had first known or suspected recurrence of treated breast cancer or stage IV metastatic breast cancer. Recent biopsy of lesions outside of bone and areas with high physiologic fluoroestradiol F 18 uptake was also required [see Dosage and Administration (2.5)]. Patients concurrently using estrogen receptor modulators or fulvestrant discontinued them 60 days prior to fluoroestradiol F 18 administration. Concurrent use of aromatase inhibitors was permitted. Three image readers were blinded to all clinical information, except for the location of the largest biopsied lesion, for which pathologists independently provided an Allred score (0 to 8). The image readers scored the intensity of FES uptake on a three-point scale relative to normal biodistribution as either "decreased," "equivocal," or "increased" (1 to 3).
Image reader performance for distinguishing between ER-positive and ER-negative fluoroestradiol F 18 uptake was compared to biopsy in 85 patients. Of the 47 patients with positive biopsy (Allred score ≥ 3), 36 were positive on imaging (majority reader score = 3). Ten of 11 patients with false negative imaging had Allred scores between 3 and 6 [see Warnings and Precautions (5.1)]. Of the 38 patients with negative biopsy, all 38 were negative on imaging.
Study 2 (NCT00602043) in 13 patients showed similar results.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
CERIANNA is supplied in a 50 mL multiple-dose glass vial (NDC 72874-001-01) containing a clear, colorless injection solution at a strength of 148 MBq/mL to 3,700 MBq/mL (4 mCi/mL to 100 mCi/mL) fluoroestradiol F 18 at the end of synthesis. Each vial contains multiple doses and is enclosed in a shield container to minimize external radiation exposure.
16.2 Storage and Handling
-
17 PATIENT COUNSELING INFORMATION
Radiation Risks
Advise patients of the radiation risks of CERIANNA [see Warnings and Precautions (5.2)]. Instruct patients to drink water to ensure adequate hydration prior to administration of CERIANNA and to continue drinking and voiding frequently during the first hours following administration to reduce radiation exposure [see Dosage and Administration (2.3)].
Pregnancy
Advise a pregnant woman of the potential risks of fetal exposure to radiation doses with CERIANNA [see Use in Specific Populations (8.1)].
Lactation
Advise a lactating woman to avoid breastfeeding for 4 hours after CERIANNA administration in order to minimize radiation exposure to a breastfed infant [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 50 mL Vial Label
CERIANNA™ (fluoroestradiol F 18) Injection
148 MBq/mL to 3,700 MBq/mL (4 mCi/mL to 100 mCi/mL) @ End of Synthesis
For Intravenous Use OnlyMultiple-Dose Vial
Rx ONLYDate of manufacture:
Expiration date & time:__________; ______hr:min
Lot# _______________________________________
Volume: ________ mL
Store at 20°C to 25°C (68°F to 77°F)
Store upright in a shielded container
Do not use if cloudy or if it contains particulate matterContains: 148 MBq/mL to 3,700 MBq/mL
(4 mCi/mL to 100 mCi/mL) of no-carrier added
fluoroestradiol F 18 @ EOS;
sodium ascorbate 0.44% w/v in sodium chloride 0.9% w/v
and ethanol no more than 3.2% w/vUsual dosage: See prescribing information
CAUTION: RADIOACTIVE
MATERIALDist.by: GE Healthcare, Inc., Arlington Heights, IL 60004 USA
GE is a trademark of General Electric Company used under
trademark license.100112-0B

-
PRINCIPAL DISPLAY PANEL - 50 mL Vial Shield Label
NDC 72874-001-01
Multiple-Dose Vial
CERIANNA™ (fluoroestradiol F 18) Injection
148 MBq/mL to 3,700 MBq/mL (4 mCi/mL to 100 mCi/mL) @ EOS*
For Intravenous Use OnlySterile, Non-pyrogenic
DiagnosticDate/time of calibration: _____________; ________hr:min
Expiration date & time: _____________; ________hr:min
Volume: _______________________ mL
Lot # ________________________
Concentration: ___________mCi/mL
at time of calibrationTotal Activity: ____________mCi at
time of calibrationContains: 148 MBq/mL to 3,700 MBq/mL (4 mCi/mL to 100
mCi/mL) of no-carrier added fluoroestradiol F 18 @ EOS*;
sodium ascorbate 0.44% w/v in sodium chloride 0.9% w/v
and ethanol no more than 3.2% w/vUsual dosage: See prescribing information
Do not use if cloudy or if it contains particulate matter*EOS = End of Synthesis
CAUTION: RADIOACTIVE MATERIAL
Expires 12 hours after EOS*
Store at 20°C to 25°C (68°F to 77°F)Store upright in a shielded container
Aseptically withdraw and handle doses[18F] Half-Life = 109.8 minutes
Calculate correct dosage from date
and time of calibrationDist. by: GE Healthcare, Inc.
Arlington Heights, IL 60004 USAGE is a trademark of General Electric
Company used under trademark license.100113-0B
Rx ONLY

-
INGREDIENTS AND APPEARANCE
CERIANNA
fluoroestradiol f 18 injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72874-001 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUOROESTRADIOL F-18 (UNII: T32277KB09) (FLUOROESTRADIOL F-18 - UNII:T32277KB09) FLUOROESTRADIOL F-18 100 mCi in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM ASCORBATE (UNII: S033EH8359) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72874-001-01 50 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product 05/20/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212155 05/20/2020 Labeler - GE Healthcare Inc. (053046579) Establishment Name Address ID/FEI Business Operations PETNET SOLUTIONS, INC. Philadelphia 004201823 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations PETNET SOLUTIONS, INC. Seattle 026659644 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations PETNET SOLUTIONS, INC. Loma Linda 079262099 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations PETNET SOLUTIONS, INC. San Francisco 080547824 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations PETNET SOLUTIONS, INC. New York 080549191 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations PETNET SOLUTIONS, INC. Raleigh-Durham 103781071 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations PETNET SOLUTIONS, INC. Jacksonville 111110727 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations PETNET SOLUTIONS, INC. South Florida 117843428 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations PETNET SOLUTIONS, INC. Chicago NW 118120165 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations PETNET SOLUTIONS, INC. Phoenix 603833208 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations PETNET SOLUTIONS, INC. Dallas 799246256 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations PETNET SOLUTIONS, INC. Atlanta 961592982 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations PETNET SOLUTIONS, INC. Louisville 961593337 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations PETNET SOLUTIONS, INC. Cleveland 961597213 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations PETNET SOLUTIONS, INC. Minneapolis 965557486 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations Kreitchman PET Center 010861487 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations P.E.T.NET HOUSTON, LLC 621380547 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations PETNET SOLUTIONS, INC. Tampa 788930480 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001) Establishment Name Address ID/FEI Business Operations PETNET SOLUTIONS, INC. (Woburn, MA) 961597122 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(72874-001)


