Label: DIANEAL LOW CALCIUM WITH DEXTROSE- sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solution
-
NDC Code(s):
0941-0711-01,
0941-0711-06,
0941-0713-01,
0941-0713-06, view more0941-0715-01, 0941-0715-06
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Health Care Provider Letter
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
BaxterLogo
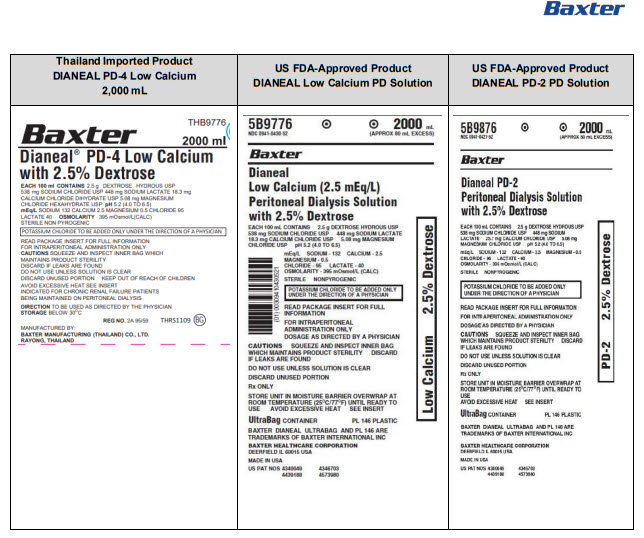
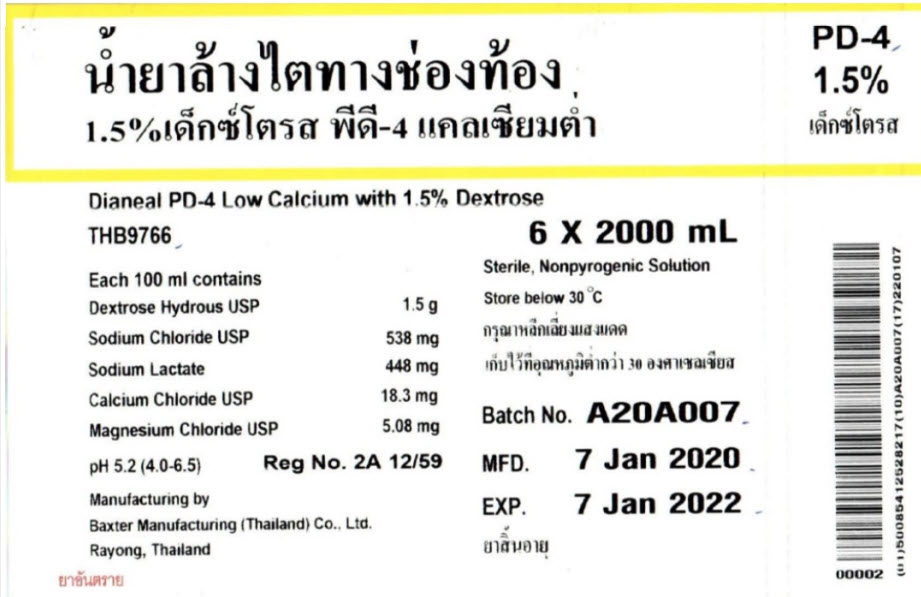
THB9766
2000 mlDianeal® PD-4 Low Calcium
with 1.5% DextroseEACH 100ml CONTAINS1.5 g DEXTROSE HYDROUS USP
538 mg SODIUM CHLORIDE USP 448 mg SODIUM LACTATE
18.3 mg CALCIUM CHLORIDE USP
5.08 mg MAGNESIUM CHLORIDE USP
pH5.2 (4.0 to 6.5)
mEq/LSODIUM 132 CALCIUM 2.5 MAGNESIUM 0.5 CHLORIDE 95
LACTATE 40 OSMOLARITY344 mOsmol/L(CALC)
STERILE NON PYROGENICPOTASSIUM CHLORIDE TO BE ADDED ONLY UNDER THE DIRECTION OF A PHYSICIAN
READ PACKAGE INSERT FOR FULL INFORMATION
FOR INTRAPERITONEAL ADMINISTRATION ONLY
CAUTIONSSQUEEZE AND INSPECT INNER BAG WHICH
MAINTAINS PRODUCT STERILITY
DISCARD IF LEAKS ARE FOUND
DO NOT USE UNLESS SOLUTION IS CLEAR
DISCARD UNUSED PORTIONKEEP OUT OF REACH OF CHILDREN
AVOID EXCESSIVE HEAT SEE INSERT
INDICATED FOR CHRONIC RENAL FAILURE PATIENTS
BEING MAINTAINED ON PERITONEAL DIALYSISDIRECTIONTO BE USED AS DIRECTED BY THE PHYSICIAN
STORAGEBELOW 30°C
REG NO.2A 12/59
THRS1107
BH Symbol
MANUFACTURED BY:
BAXTER MANUFACTURING (THAILAND) CO. LTD.
RAYONG, THAILANDPD-4
1.5%Dianeal PD-4 Low Calcium with 1.5% Dextrose
THB9766
6 X 2000 mLEach 100 ml contains
Dextrose Hydrous USP 1.5 g
Sodium Chloride USP 538 mg
Sodium Lactate 448 mg
Calcium Chloride USP 18.3 mg
Magnesium Chloride USP 5.08 mg
pH 5.2 (4.0-6.5)Reg No. 2A 12/59
Manufacturing by
Baxter Manufacturing (Thailand) Co., Ltd.
Rayong, ThailandSterile, Nonpyrogenic Solution
Store below 30°CBatch No. A20A007
MFD. 7 Jan 2020
EXP. 7 Jan 2022Barcode
00002(01)50085412528217(10)A20A007(17)220107
BaxterLogo
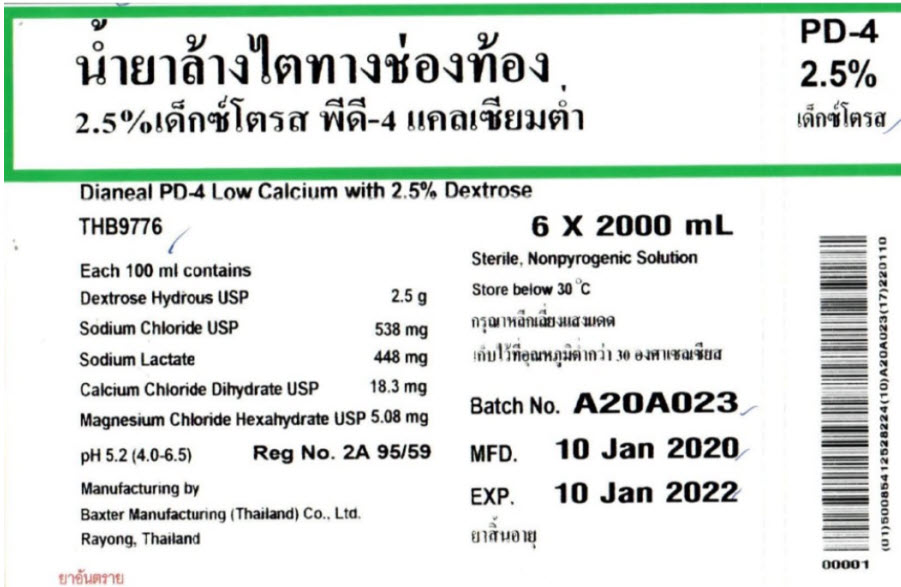
THB9776
2000 mlDianeal® PD-4 Low Calcium
with 2.5% DextroseEACH 100 ml CONTAINS2.5 g DEXTROSE HYDROUS USP
538 mg SODIUM CHLORIDE USP 448 mg SODIUM LACTATE 18.3 mg
CALCIUM CHLORIDE DIHYDRATE USP 5.08 mg MAGNESIUM
CHLORIDE HEXAHYDRATE USP pH5.2 (4.0 to 6.5)
mEq/LSODIUM 132 CALCIUM 2.5 MAGNESIUM 0.5 CHLORIDE 95
LACTATE 40 OSMOLARITY395 mOsmol/L(CALC)
STERILE NON PYROGENICPOTASSIUM CHLORIDE TO BE ADDED ONLY UNDER THE DIRECTION OF A PHYSICIAN
READ PACKAGE INSERT FOR FULL INFORMATION
FOR INTRAPERITONEAL ADMINISTRATION ONLY
CAUTIONSSQUEEZE AND INSPECT INNER BAG WHICH
MAINTAINS PRODUCT STERILITY
DISCARD IF LEAKS ARE FOUND
DO NOT USE UNLESS SOLUTION IS CLEAR
DISCARD UNUSED PORTION KEEP OUT OF REACH OF CHILDRENAVOID EXCESSIVE HEAT SEE INSERT
INDICATED FOR CHRONIC RENAL FAILURE PATIENTS
BEING MAINTAINED ON PERITONEAL DIALYSISDIRECTIONTO BE USED AS DIRECTED BY THE PHYSICIAN
STORAGEBELOW 30°CREG NO.2A 95/59
THRS1109
BG SymbolMANUFACTURED BY:
BAXTER MANUFACTURING (THAILAND) CO. LTD.
RAYONG, THAILANDPD-4
2.5%Dianeal PD-4 Low Calcium with 2.5% Dextrose
THB9776
6 X 2000 mLEach 100 ml contains
Dextrose Hydrous USP 2.5 g
Sodium Chloride USP 538 mg
Sodium Lactate 448 mg
Calcium Chloride Dihydrate USP 18.3 mg
Magnesium Chloride Hexahydrate USP 5.08 mgpH 5.2 (4.0-6.5)
Reg No. 2A 95/59
Manufacturing by
Baxter Manufacturing (Thailand) Co., Ltd.
Rayong, ThailandSterile, Nonpyrogenic Solution
Store below 30°CBatch No. A20A023
MFD. 10 Jan 2020
EXP. 10 Jan 2022Barcode
00001(01)500854 12528224(10)A20A023(17)220110
BaxterLogo
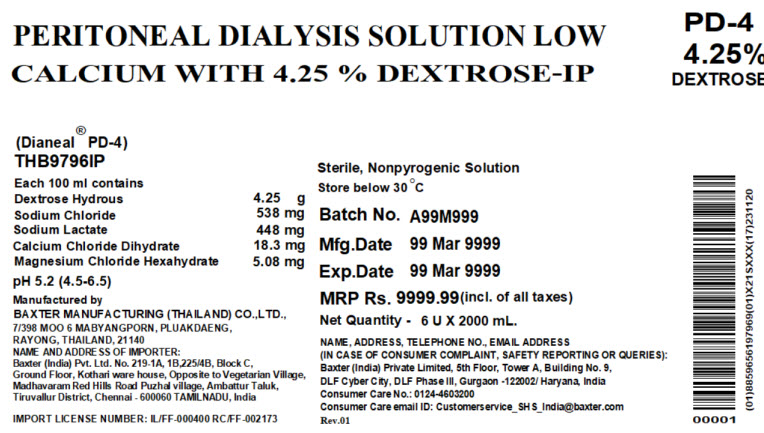
THB9796IP
2000 mlPeritoneal Dialysis Solution Low Calcium with
4.5% Dextrose-IP
Dianeal® PD-4 Low CalciumEACH 100 ml CONTAINS4.25 g DEXTROSE HYDROUS 538 mg SODIUM CHLORIDE
448 mg SODIUM LACTATE 18.3 mg CALCIUM CHLORIDE DIHYDRATE 5.08 mg
MAGNESIUM CHLORIDE HEXAHYDRATE pH5.2 (4.5 to 6.5) mEq/L
SODIUM 132 CALCIUM 2.5 MAGNESIUM 0.5 CHLORIDE 95 LACTATE 40
OSMOLARITY483 mOsmol/L(CALC) STERILE NON PYROGENICPOTASSIUM CHLORIDE TO BE ADDED ONLY UNDER THE DIRECTION OF A PHYSICIAN
FOR INTRAPERITONEAL ADMINISTRATION ONLY
CAUTIONSSQUEEZE AND INSPECT INNER BAG WHICH
MAINTAINS PRODUCT STERILITY DISCARD IF LEAKS ARE FOUND
DO NOT USE UNLESS SOLUTION IS CLEAR DISCARD UNUSED PORTION
KEEP OUT OF REACH OF CHILDREN AVOID EXCESSIVE HEAT
INDICATED FOR CHRONIC RENAL FAILURE PATIENTS
BEING MAINTAINED ON PERITONEAL DIALYSISINDICATION/ADMINISTRATION AND COMPLETE INFORMATION –REFER PACKAGE INSERT
STORAGEBELOW 30°C
THA REG NO.2A 93/59
THRS1131
AA SymbolNAME AND ADDRESS OF MANUFACTURER:
BAXTER MANUFACTURING (THAILAND) CO., LTD.
7/398 MOO 6 MABYANGPORN, PLUAKDAENG, RAYONG, THAILAND 21140Name and Address of Importer:
Baxter (India) Pvt. Ltd., No. 219-1A, 1B,225/4B, Block C, Ground Floor,
Kothari ware house, Opposite to Vegetarian Village, Madhavaram
Red Hills Road Puzhal village, Ambattur Taluk, Tiruvallur District,
Chennai – 600060 Tamil Nadu, IndiaImport License Number:IL/FF-000400 RC/FF-002173
Telephone no., email address (in case of consumer complaint, Safety Reporting or Queries):
Consumer Care No.: 0124-4603200
Consumer Care email ID: Customerservice_SHS_India@baxter.comPEITONEAL DIALYSIS SOLUTION LOW
CALCIUM WITH 4.25% DEXTROSE-IPPD-4
4.25%
DEXTROSE(Dianeal® PD-4)
THB9796IPEach 100 ml contains
Dextrose Hydrous 4.25 g
Sodium Chloride 538 mg
Sodium Lactate 448 mg
Calcium Chloride Dihydrate 18.3 mg
Magnesium Chloride Hexahydrate 5.08 mgpH 5.2 (4.5-6.5)
Manufactured by
BAXTER MANUFACTURING (THAILAND) CO., LTD.,
7/398 MOO 6 MABYANGPORN, PLUAKDAENG,
RAYONG, THAILAND, 21140
NAME AND ADDRESS OF IMPORTER:
Baxter (India) Pvt. Ltd. No. 219-1A, 1B,225/4B, Block C,
Ground Floor, Kothari ware house, Opposite to Vegetarian Village,
Madhavaran Red Hills Road Puzhal village, Ambattur Taluk,
Tiruvallur District, Chennai – 600060 TAMILNADU, IndiaIMPORT LICENSE NUMBER: IL/FF-000400 RC/RF-002173
Sterile, Nonpyrogenic Solution
Store below 30°CBatch No. A99A999
MFD. 99 Mar 9999
EXP. 99 Mar 9999MRP Rs. 9999.99(incl. of all taxes)
Net Quality – 6 U X 2000 mL.NAME, ADDRESS, TELEPHONE NO., EMAIL ADDRESS
(IN CASE OF CONSUMER COMPLAINT, SAFETY REPORTING OR QUERIES):
Baxter (India) Private Limited, 5 thFloor, Tower A, Building No. 9,
DLF Cyber City, DLF Phase III, Gurgaon – 122002/ Haryana, India
Consumer Care No.: 0124-4603200
Consumer Care email ID: Customerservice_SHS_India@baxter.com
Rev.01Barcode
00001(01)8859656197969(10)X21SXXX(17)231120
-
INGREDIENTS AND APPEARANCE
DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0941-0711 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 1.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.3 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0941-0711-06 6 in 1 CARTON 10/23/2024 1 NDC:0941-0711-01 2000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/23/2024 DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0941-0713 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 2.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.3 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0941-0713-06 6 in 1 CARTON 10/23/2024 1 NDC:0941-0713-01 2000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/23/2024 DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0941-0715 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 4.25 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.3 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0941-0715-06 6 in 1 CARTON 10/23/2024 1 NDC:0941-0715-01 2000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/23/2024 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Manufacturing (Thailand) Co., Ltd 661742706 analysis(0941-0711, 0941-0713, 0941-0715) , label(0941-0711, 0941-0713, 0941-0715) , manufacture(0941-0711, 0941-0713, 0941-0715) , pack(0941-0711, 0941-0713, 0941-0715) , sterilize(0941-0711, 0941-0713, 0941-0715)