Label: PERFECT BUSTY BOOSTER- chlorella vulgaris, pcydonia oblonga, garcinia mangostana cream
- NDC Code(s): 71326-301-11, 71326-301-51
- Packager: HOT PRODUCTIONS AND VERTRIEBS GMBH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
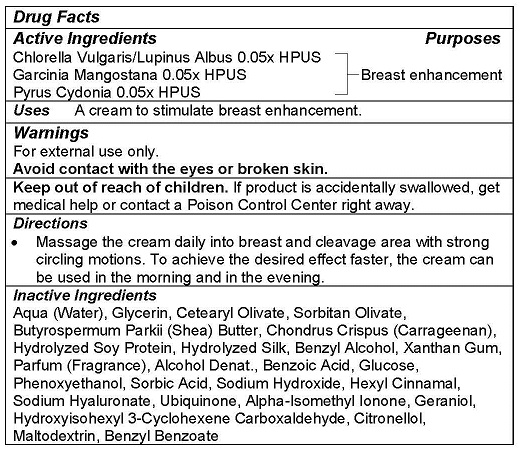
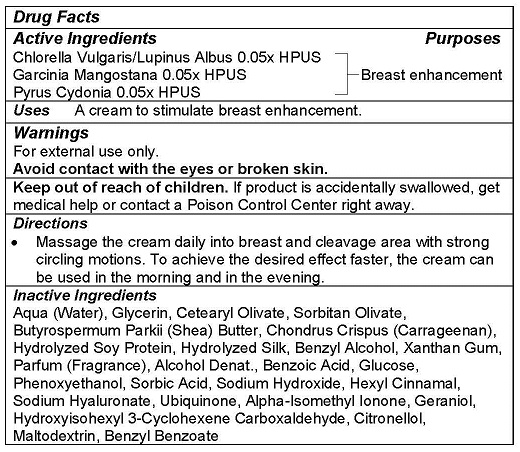
- ACTIVE INGREDIENTS
- PURPOSE
- USE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
-
INACTIVE INGREDIENTS
Aqua (Water), Glycerin, Cetearyl Olivate, Sorbitan Olivate, Butyrospermum Parkii (Shea) Butter, Chondrus Crispus (Carrageenan), Hydrolyzed Soy Protein, Hydrolyzed Silk, Benzyl Alcohol, Xanthan Gum, Parfum (Fragrance), Alcohol Denat., Benzoic Acid, Glucose, Phenoxyethanol, Sorbic Acid, Sodium Hydroxide, Hexyl Cinnamal, Sodium Hyaluronate, Ubiquinone, Alpha-Isomethyl Ionone, Geraniol, Hydroxyisohexyl 3-Cyclohexene Carboxaldehyde, Citronellol, Maltodextrin, Benzyl Benzoate
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PERFECT BUSTY BOOSTER
chlorella vulgaris, pcydonia oblonga, garcinia mangostana creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71326-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORELLA VULGARIS (UNII: RYQ4R60M02) (CHLORELLA VULGARIS - UNII:RYQ4R60M02) CHLORELLA VULGARIS 0.05 g in 100 mL CYDONIA OBLONGA SEED (UNII: JXU526QH1V) (CYDONIA OBLONGA SEED - UNII:JXU526QH1V) CYDONIA OBLONGA SEED 0.05 g in 100 mL GARCINIA MANGOSTANA FRUIT RIND (UNII: 1340BFH77T) (GARCINIA MANGOSTANA FRUIT RIND - UNII:1340BFH77T) GARCINIA MANGOSTANA FRUIT RIND 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CETEARYL OLIVATE (UNII: 58B69Q84JO) SORBITAN OLIVATE (UNII: MDL271E3GR) SHEA BUTTER (UNII: K49155WL9Y) BENZYL ALCOHOL (UNII: LKG8494WBH) CHONDRUS CRISPUS CARRAGEENAN (UNII: UE856F2T78) XANTHAN GUM (UNII: TTV12P4NEE) ALCOHOL (UNII: 3K9958V90M) HYDROLYZED SOY PROTEIN (ENZYMATIC; 2000 MW) (UNII: 1394NXB9L6) BENZOIC ACID (UNII: 8SKN0B0MIM) AMINO ACIDS, SILK (UNII: V0L00EX1IA) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) PHENOXYETHANOL (UNII: HIE492ZZ3T) SORBIC ACID (UNII: X045WJ989B) SODIUM HYDROXIDE (UNII: 55X04QC32I) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) HYALURONATE SODIUM (UNII: YSE9PPT4TH) UBIDECARENONE (UNII: EJ27X76M46) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) GERANIOL (UNII: L837108USY) HYDROXYISOHEXYL 3-CYCLOHEXENE CARBOXALDEHYDE (UNII: QUE43B9Z2Q) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) MALTODEXTRIN (UNII: 7CVR7L4A2D) BENZYL BENZOATE (UNII: N863NB338G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71326-301-51 1 in 1 BOX 08/30/2018 1 NDC:71326-301-11 100 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/30/2018 Labeler - HOT PRODUCTIONS AND VERTRIEBS GMBH (300011984)