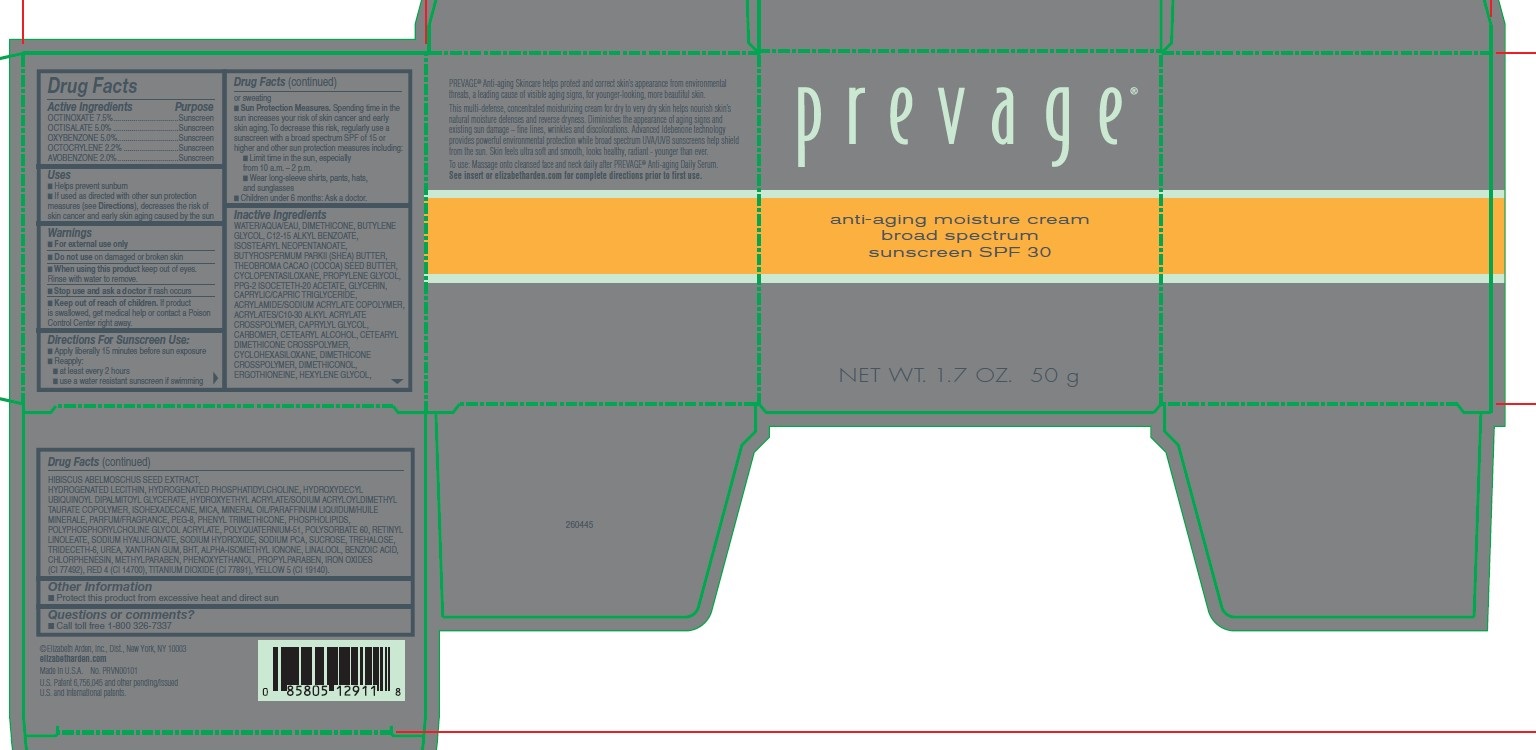
Label: PREVAGE ANTI-AGING MOISTURE CREAM- octinoxate, octisalate, oxybenzone, octocrylene, avobenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 10967-688-17 - Packager: Revlon Consumer Products Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 9, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Inactive ingredients
WATER/AQUA/EAU, DIMETHICONE, BUTYLENE GLYCOL, C12-15 ALKYL BENZOATE, ISOSTEARYL NEOPENTANOATE, BUTYROSPERMUM PARKII (SHEA) BUTTER, THEOBROMA CACAO (COCOA) SEED BUTTER, CYCLOPENTASILOXANE, PROPYLENE GLYCOL, PPG-2 ISOCETETH-20 ACETATE, GLYCERIN, CAPRYLIC/CAPRIC TRIGLYCERIDE, ACRYLAMIDE/SODIUM ACRYLATE COPOLYMER, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, CAPRYLYL GLYCOL, CARBOMER, CETEARYL ALCOHOL, CETEARYL DIMETHICONE CROSSPOLYMER, CYCLOHEXASILOXANE, DIMETHICONE CROSSPOLYMER, DIMETHICONOL, ERGOTHIONEINE, HEXYLENE GLYCOL, HIBISCUS ABELMOSCHUS SEED EXTRACT, HYDROGENATED LECITHIN, HYDROGENATED PHOSPHATIDYLCHOLINE, HYDROXYDECYL UBIQUINOYL DIPALMITOYL GLYCERATE, HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER, ISOHEXADECANE, MICA, MINERAL OIL/PARAFFINUM LIQUIDUM/HUILE MINERALE, PARFUM/FRAGRANCE, PEG-8, PHENYL TRIMETHICONE, PHOSPHOLIPIDS, POLYPHOSPHORYLCHOLINE GLYCOL ACRYLATE, POLYQUATERNIUM-51, POLYSORBATE 60, RETINYL LINOLEATE, SODIUM HYALURONATE, SODIUM HYDROXIDE, SODIUM PCA, SUCROSE, TREHALOSE, TRIDECETH-6, UREA, XANTHAN GUM, BHT, ALPHA-ISOMETHYL IONONE, LINALOOL, BENZOIC ACID, CHLORPHENESIN, METHYLPARABEN, PHENOXYETHANOL, PROPYLPARABEN, IRON OXIDES (CI 77492), RED 4 (CI 14700), TITANIUM DIOXIDE (CI 77891), YELLOW 5 (CI 19140)
- Active Ingredient Section
- Warnings
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PREVAGE ANTI-AGING MOISTURE CREAM
octinoxate, octisalate, oxybenzone, octocrylene, avobenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-688 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 5 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2.2 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 mg in 1 g Inactive Ingredients Ingredient Name Strength HYDROXYDECYL UBIQUINOYL DIPALMITOYL GLYCERATE (UNII: OV1BT2N8RC) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) ISOHEXADECANE (UNII: 918X1OUF1E) MICA (UNII: V8A1AW0880) SHEA BUTTER (UNII: K49155WL9Y) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) CHLORPHENESIN (UNII: I670DAL4SZ) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARBOMER 1342 (UNII: 809Y72KV36) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) HEXYLENE GLYCOL (UNII: KEH0A3F75J) DIMETHICONE 1000 (UNII: MCU2324216) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) PPG-2 ISOCETETH-20 ACETATE (UNII: BI6C7YO419) CYCLOMETHICONE 6 (UNII: XHK3U310BA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) DIMETHICONOL (100000 CST) (UNII: OSA9UP217S) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) TRIDECETH-6 (UNII: 3T5PCR2H0C) COCOA BUTTER (UNII: 512OYT1CRR) CARBOMER INTERPOLYMER TYPE A (55000 CPS) (UNII: 59TL3WG5CO) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERIN (UNII: PDC6A3C0OX) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) PEG-9 DIGLYCIDYL ETHER/SODIUM HYALURONATE CROSSPOLYMER (UNII: 788QAG3W8A) TREHALOSE (UNII: B8WCK70T7I) SODIUM HYDROXIDE (UNII: 55X04QC32I) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) ACRYLAMIDE (UNII: 20R035KLCI) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ERGOTHIONEINE (UNII: BDZ3DQM98W) XANTHAN GUM (UNII: TTV12P4NEE) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) BENZOIC ACID (UNII: 8SKN0B0MIM) PHENOXYETHANOL (UNII: HIE492ZZ3T) UREA (UNII: 8W8T17847W) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) CETYL DIMETHICONE 150 (UNII: 5L694Y0T22) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ABELMOSCHUS MOSCHATUS SEED (UNII: UN2QZ55I88) POLYSORBATE 60 (UNII: CAL22UVI4M) RETINYL LINOLEATE (UNII: 61911N8D6W) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-688-17 1 g in 1 JAR; Type 0: Not a Combination Product 01/02/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 01/02/2020 Labeler - Revlon Consumer Products Corp (788820165) Establishment Name Address ID/FEI Business Operations ENGLEWOOD LAB, INC 080987545 manufacture(10967-688)