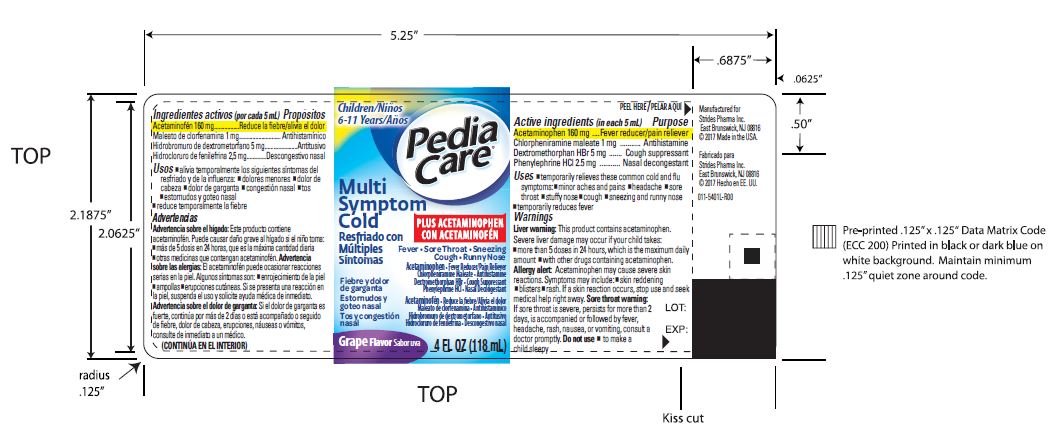
Label: PEDIACARE MULTI SYMPTOM COLD- acetaminophen,chlorpheniramine maleate,dextromethorphan hbr,phenylephrine hcl liquid
- NDC Code(s): 59556-851-58
- Packager: Strides Pharma Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 7, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purposes
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Sever liver damage may occur if your child takes:
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- to make a child sleepy
- in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if the child is allergic to any of the ingredients in this product
Ask a doctor before use if the child has
- glaucoma
- thyroid disease
- diabetes
- high blood pressure
- heart disease
- a breathing problem such as chronic bronchitis
- persistent or chronic cough such as occurs with asthma
- cough that occurs with excessive phlegm (mucus)
- liver disease
Ask a doctor or pharmacist before use if child is
- taking sedatives or tranquilizers
- taking the blood thinning drug warfarin
- do not exceed recommended dosage (see overdose warning)
- may cause excitability, especially in children
- marked drowsiness may occur
- sedatives and tranquilizers may increase drowsiness
- nervousness, dizziness or sleeplessness occur
- pain, nasal congestion or cough gets worse or lasts more than 5 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
- cough comes back or occurs with rash or headache that lasts.These could be signs of a serious condition.
-
Keep out of reach of children
Overdose Warning: Taking more than the recommended dose (overdose) could cause serious health problems, including liver damage. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away. Quick medical attention is critical even if you do not notice any signs or symptoms.
-
Directions
- do not take more than directed (see overdose warning)
- do not use in infants
- this product does not contain directions or complete warnings for adult use
- shake well before using
- use only the provided dosing cup
- find right dose on chart below. If possible, use weight to dose; otherwise, use age
- if needed, repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
- mL = millilitre
Weight (lbs)
Age (yrs)
Dose (mL)
under 36
under 4
do not use
36 - 47
4 to under 6
do not use unless directed by doctor
48 - 95
6 - 11
10 mL
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PEDIACARE MULTI SYMPTOM COLD
acetaminophen,chlorpheniramine maleate,dextromethorphan hbr,phenylephrine hcl liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59556-851 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg in 5 mL CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 1 mg in 5 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 5 mg in 5 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 2.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color Score Shape Size Flavor GRAPE (Grape Flavor) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59556-851-58 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 07/09/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 07/09/2010 Labeler - Strides Pharma Inc (078868278) Establishment Name Address ID/FEI Business Operations Fareva Richmond, Inc. 969523245 MANUFACTURE(59556-851) , ANALYSIS(59556-851) , LABEL(59556-851) , PACK(59556-851)