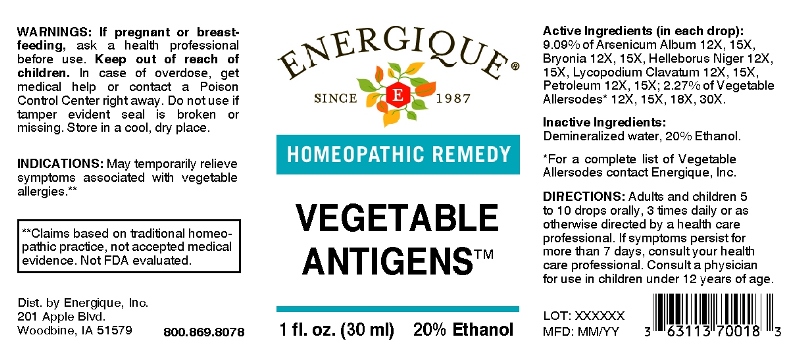
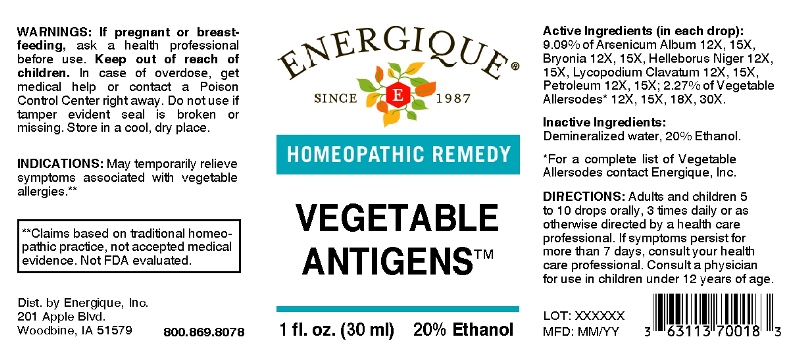
Label: VEGETABLE ANTIGENS (allium cepa, allium sativum, apium graveolens, beta vulgaris, brassica oleracea var. botrytis, brassica oleracea var. capitata, brassica oleracea var. italica, brassica oleracea var. sabellica, brassica rapa var. rapa, cicer arietinum, cucumis staivus, glycine max, lactuca sativa, lens culinaris, pastinaca sativa, phaseolus, phaseolus lunatus, phaseolus vulgaris, raphanus sativus, spinacia oleracea, arsenicum album, bryonia- alba, helleborus niger, lycopodium clavatum, petroleum liquid
- NDC Code(s): 44911-0361-1
- Packager: Energique, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated June 30, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS:
(in each drop) : 9.09% of Arsenicum Album 12X, 15X, Bryonia (Alba) 12X, 15X, Helleborus Niger 12X, 15X, Lycopodium Clavatum 12X, 15X, Petroleum 12X, 15X; 2.27% of Allium Cepa 12X, 15X, 18X, 30X, Allium Sativum 12X, 15X, 18X, 30X, Apium Graveolens 12X, 15X, 18X, 30X, Beta Vulgaris 12X, 15X, 18X, 30X, Brassica Oleracea Var. Botrytis 12X, 15X, 18X, 30X, Brassica Oleracea Var. Capitata 12X, 15X, 18X, 30X, Brassica Oleracea Var. Italica 12X, 15X, 18X, 30X, Brassica Oleracea Var. Sabellica 12X, 15X, 18X, 30X, Brassica Rapa Var. Rapa 12X, 15X, 18X, 30X, Cicer Arietinum 12X, 15X, 18X, 30X, Cucumis Sativus 12X, 15X, 18X, 30X, Glycine Max 12X, 15X, 18X, 30X, Lactuca Sativa 12X, 15X, 18X, 30X, Lens Culinaris 12X, 15X, 18X, 30X, Pastinaca Sativa 12X, 15X, 18X, 30X, Phaseolus 12X, 15X, 18X, 30X, Phaseolus Lunatus 12X, 15X, 18X, 30X, Phaseolus Vulgaris 12X, 15X, 18X, 30X, Raphanus Sativus 12X, 15X, 18X, 30X, Spinacia Oleracea 12X, 15X, 18X, 30X.
- INDICATIONS:
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INDICATIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
VEGETABLE ANTIGENS
allium cepa, allium sativum, apium graveolens, beta vulgaris, brassica oleracea var. botrytis, brassica oleracea var. capitata, brassica oleracea var. italica, brassica oleracea var. sabellica, brassica rapa var. rapa, cicer arietinum, cucumis staivus, glycine max, lactuca sativa, lens culinaris, pastinaca sativa, phaseolus, phaseolus lunatus, phaseolus vulgaris, raphanus sativus, spinacia oleracea, arsenicum album, bryonia (alba), helleborus niger, lycopodium clavatum, petroleum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:44911-0361 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 12 [hp_X] in 1 mL GARLIC (UNII: V1V998DC17) (GARLIC - UNII:V1V998DC17) GARLIC 12 [hp_X] in 1 mL CELERY SEED (UNII: 1G1EAA320L) (CELERY SEED - UNII:1G1EAA320L) CELERY SEED 12 [hp_X] in 1 mL BEET (UNII: N487KM8COK) (BEET - UNII:N487KM8COK) BEET 12 [hp_X] in 1 mL CAULIFLOWER (UNII: 138LUT2DWV) (CAULIFLOWER - UNII:138LUT2DWV) CAULIFLOWER 12 [hp_X] in 1 mL CABBAGE (UNII: GW0W1Y9I97) (CABBAGE - UNII:GW0W1Y9I97) CABBAGE 12 [hp_X] in 1 mL BROCCOLI (UNII: UOI4FT57BZ) (BROCCOLI - UNII:UOI4FT57BZ) BROCCOLI 12 [hp_X] in 1 mL KALE (UNII: 0Y3L4J38H1) (KALE - UNII:0Y3L4J38H1) KALE 12 [hp_X] in 1 mL TURNIP (UNII: Z38C7FBM49) (TURNIP - UNII:Z38C7FBM49) TURNIP 12 [hp_X] in 1 mL CHICKPEA (UNII: N91637DNW9) (CHICKPEA - UNII:N91637DNW9) CHICKPEA 12 [hp_X] in 1 mL CUCUMBER (UNII: YY7C30VXJT) (CUCUMBER - UNII:YY7C30VXJT) CUCUMBER 12 [hp_X] in 1 mL SOYBEAN (UNII: L7HT8F1ZOD) (SOYBEAN - UNII:L7HT8F1ZOD) SOYBEAN 12 [hp_X] in 1 mL LETTUCE (UNII: 5PO6NN3RRJ) (LETTUCE - UNII:5PO6NN3RRJ) LETTUCE 12 [hp_X] in 1 mL LENTIL (UNII: 6O38V6B52O) (LENTIL - UNII:6O38V6B52O) LENTIL 12 [hp_X] in 1 mL PARSNIP (UNII: L2V28YP49S) (PARSNIP - UNII:L2V28YP49S) PARSNIP 12 [hp_X] in 1 mL KIDNEY BEAN (UNII: M98C8416QO) (KIDNEY BEAN - UNII:M98C8416QO) KIDNEY BEAN 12 [hp_X] in 1 mL LIMA BEAN (UNII: 112YH1ZMX2) (LIMA BEAN - UNII:112YH1ZMX2) LIMA BEAN 12 [hp_X] in 1 mL STRING BEAN (UNII: N9D69B2Q7Y) (STRING BEAN - UNII:N9D69B2Q7Y) STRING BEAN 12 [hp_X] in 1 mL RADISH (UNII: EM5RP35463) (RADISH - UNII:EM5RP35463) RADISH 12 [hp_X] in 1 mL SPINACH (UNII: 6WO75C6WVB) (SPINACH - UNII:6WO75C6WVB) SPINACH 12 [hp_X] in 1 mL ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 12 [hp_X] in 1 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 12 [hp_X] in 1 mL HELLEBORUS NIGER ROOT (UNII: 608DGJ6815) (HELLEBORUS NIGER ROOT - UNII:608DGJ6815) HELLEBORUS NIGER ROOT 12 [hp_X] in 1 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 12 [hp_X] in 1 mL KEROSENE (UNII: 1C89KKC04E) (KEROSENE - UNII:1C89KKC04E) KEROSENE 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44911-0361-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 06/01/2016 02/10/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/01/2016 02/10/2025 Labeler - Energique, Inc. (789886132) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(44911-0361) , api manufacture(44911-0361) , label(44911-0361) , pack(44911-0361)