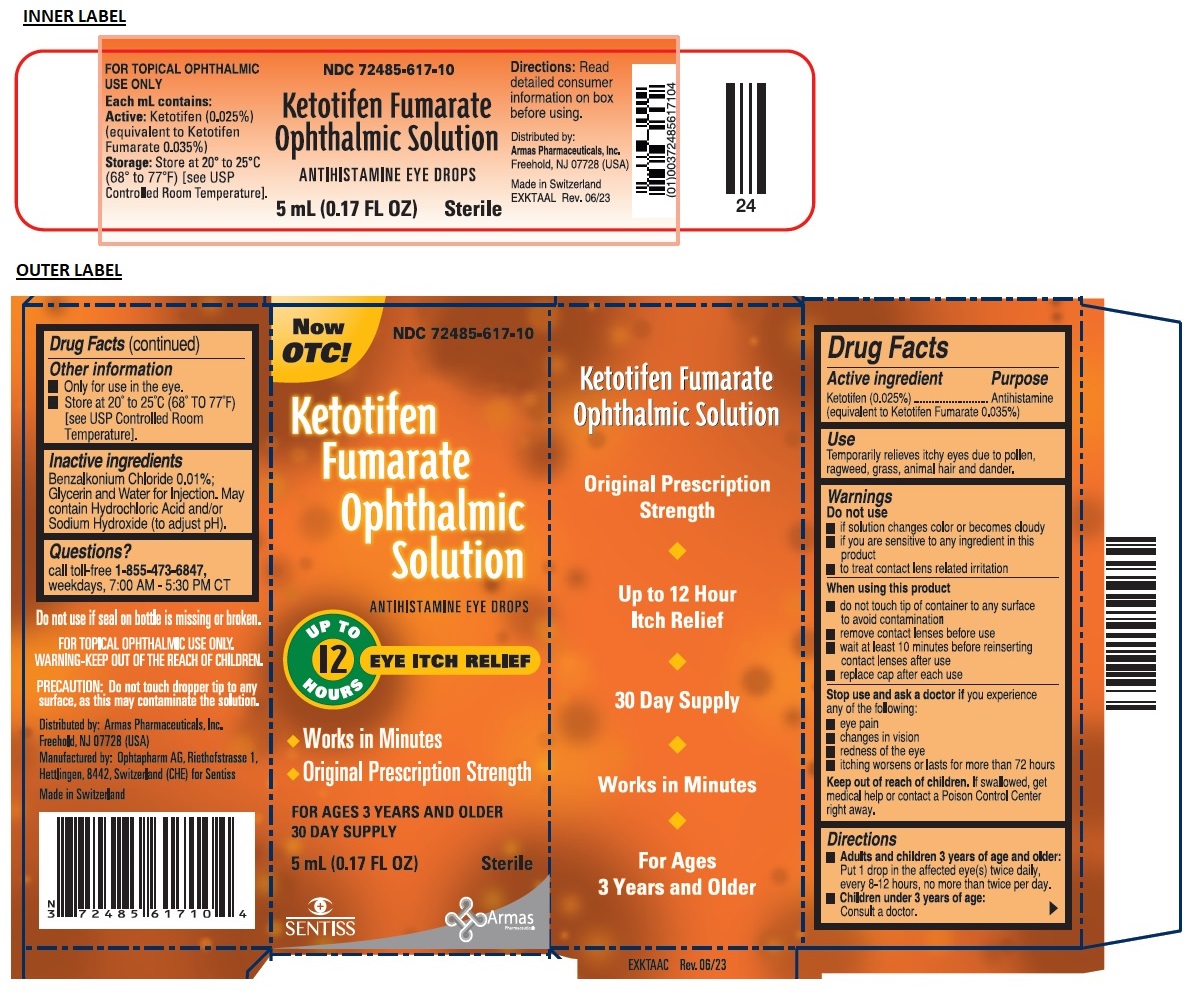
Label: KETOTIFEN FUMARATE OPHTHALMIC SOLUTION- ketotifen fumarate solution/ drops
- NDC Code(s): 72485-617-10
- Packager: Armas Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- replace cap after each use
Stop use and ask a doctor if you experience any of the following:
- eye pain
- changes in vision
- redness of the eye
- itching worsens or lasts for more than 72 hours
- Directions
- Other information
- Inactive ingredients
- Questions?
-
SPL UNCLASSIFIED SECTION
Now OTC!
ANTIHISTAMINE EYE DROPS
UPTO 12 HOURS EYE ITCH RELIEF
• Works in Minutes
• Original Prescription Strength
FOR AGES 3 YEARS AND OLDER
30 DAY SUPPLYSterile
Original Prescription Strength
Do not use if seal on bottle is missing or broken.
FOR TOPICAL OPHTHALMIC USE ONLY.
PRECAUTION: Do not touch dropper tip to any surface, as this may contaminate the solution.
Distributed by: Armas Pharmaceuticals, Inc.
Freehold, NJ 07728 (USA)Manufactured by: Ophtapharm AG, Riethofstrasse 1,
Hettlingen, 8442, Switzerland (CHE) for SentissMade in Switzerland
- Packaging
-
INGREDIENTS AND APPEARANCE
KETOTIFEN FUMARATE OPHTHALMIC SOLUTION
ketotifen fumarate solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72485-617 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength KETOTIFEN FUMARATE (UNII: HBD503WORO) (KETOTIFEN - UNII:X49220T18G) KETOTIFEN 0.25 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72485-617-10 1 in 1 CARTON 10/09/2023 1 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077958 10/09/2023 Labeler - Armas Pharmaceuticals Inc. (098405973) Registrant - SENTISS AG (486920486)