Label: THERA CARE COLD HOT MEDICATED PATCH- menthol patch
- NDC Code(s): 71101-954-05
- Packager: Veridian Healthcare
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- When using this product
- Stop use and ask a doctor
- If pregnant or breastfeeding
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
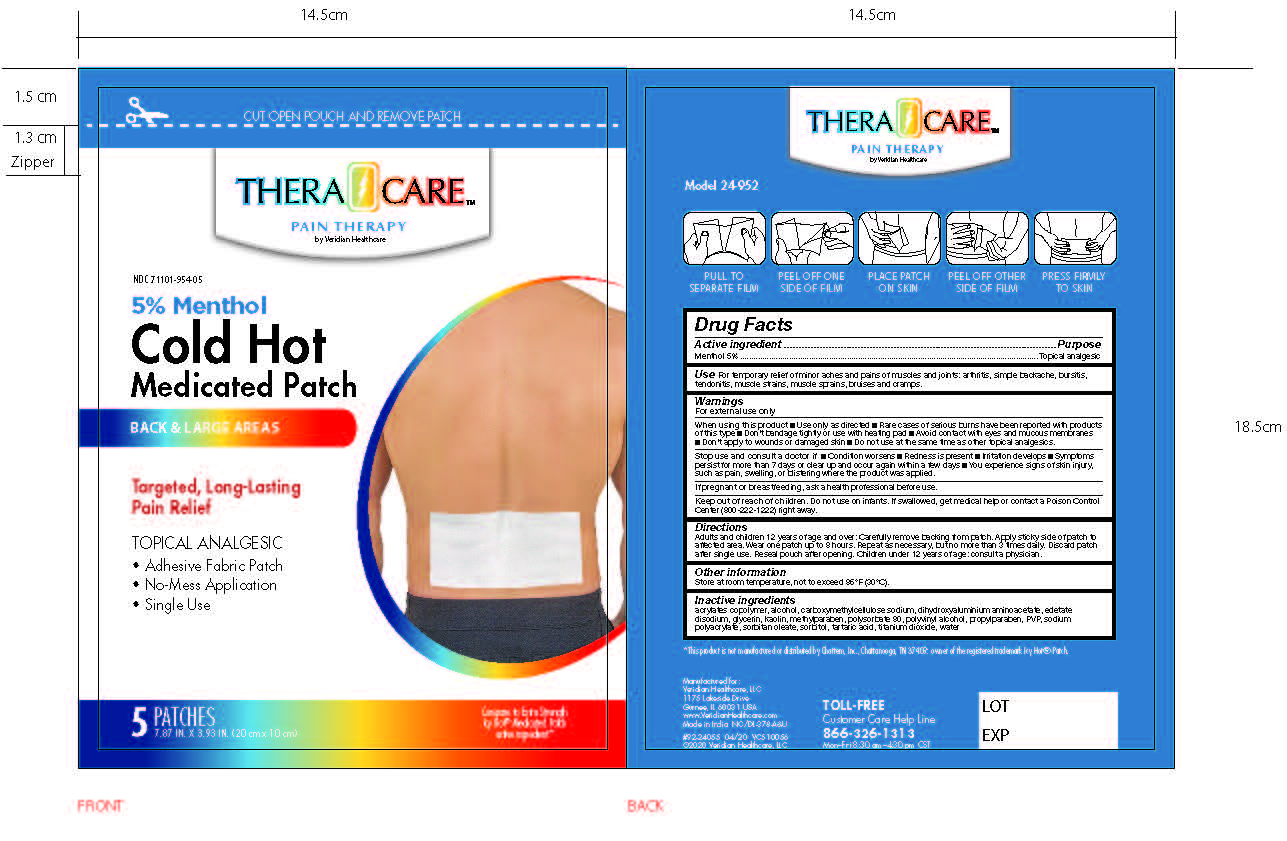
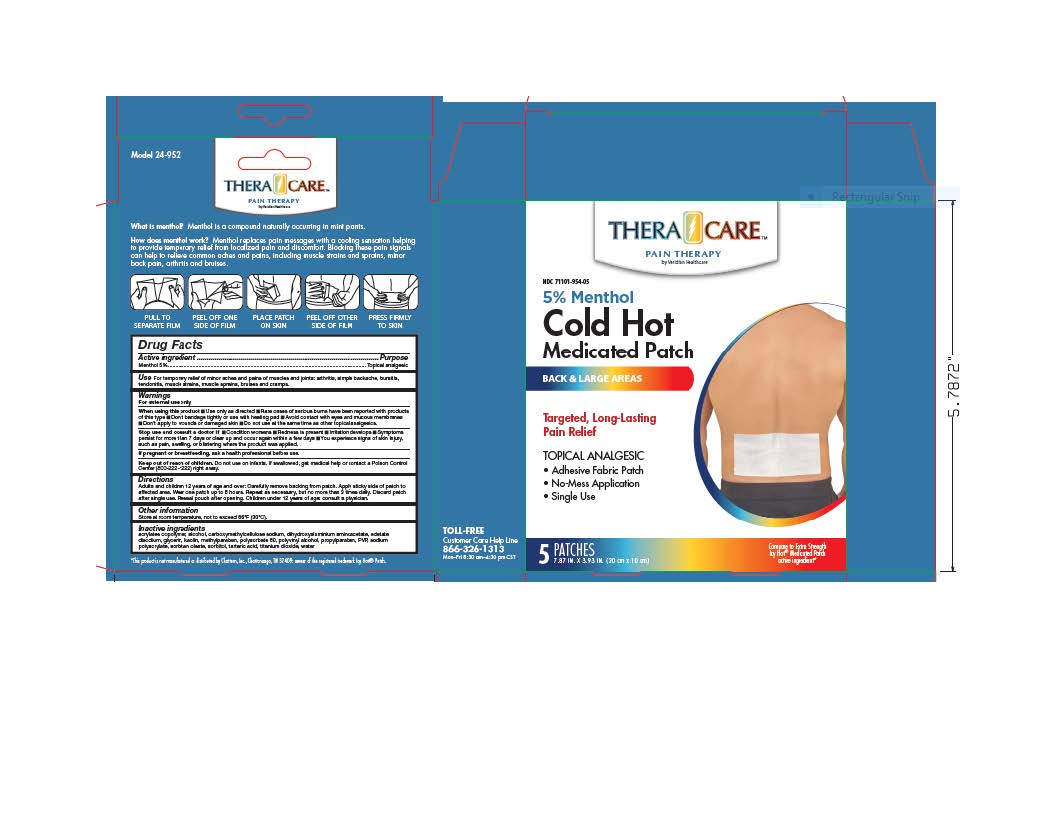
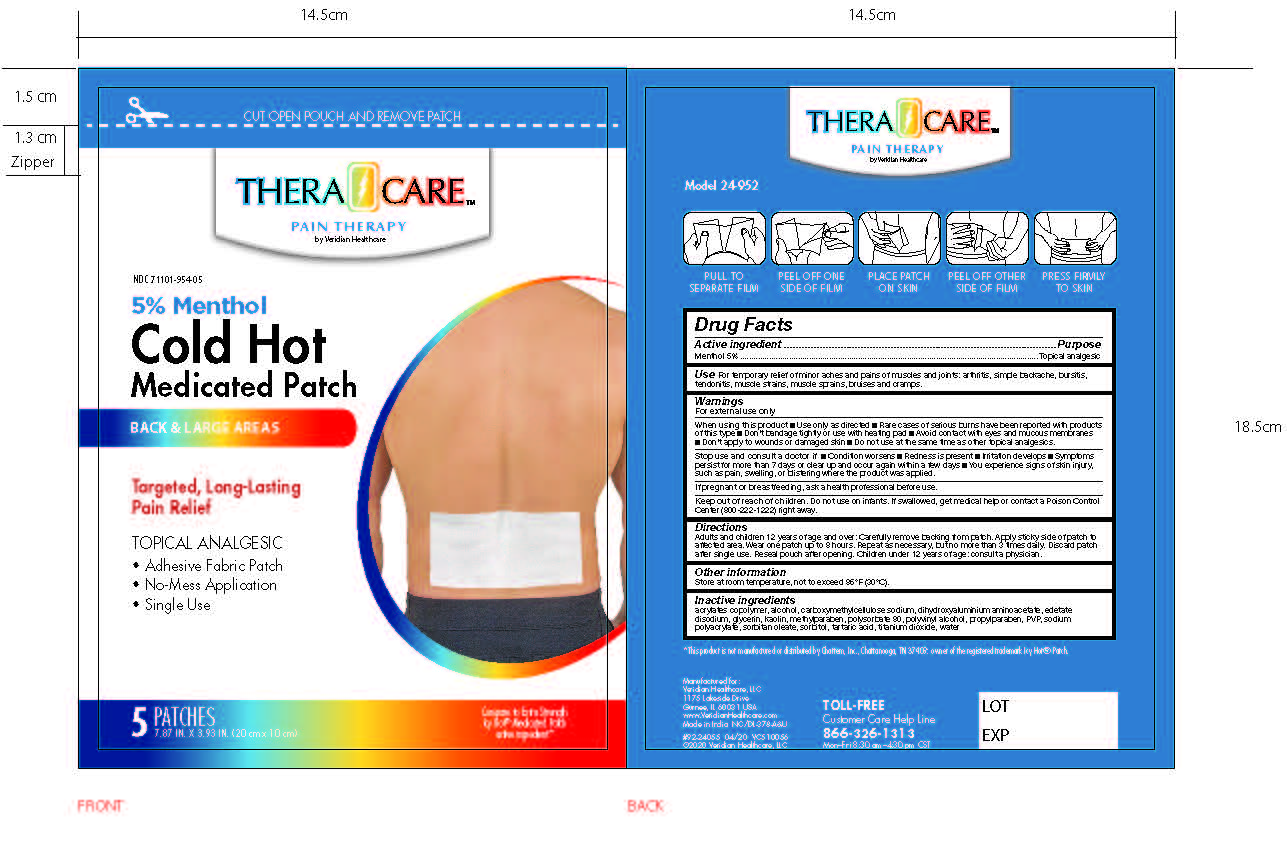
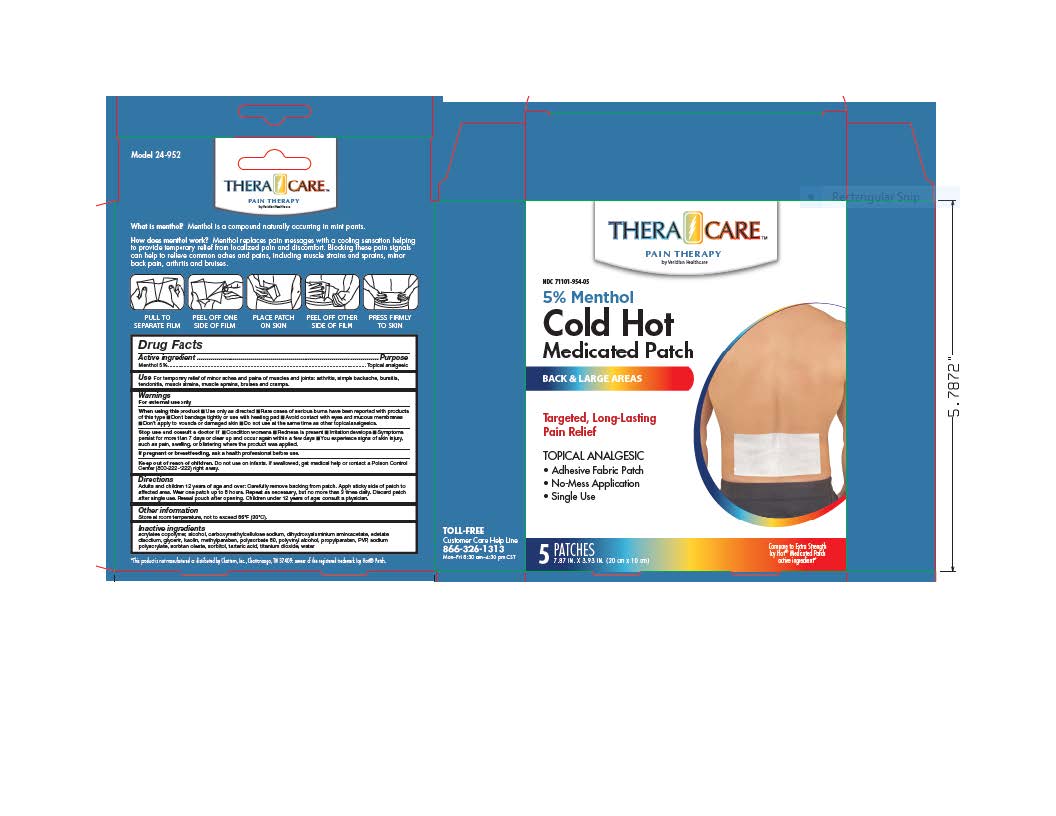
Adults and children over 12 years: Carefully remove backing from patch. Apply sticky side of patch to affected area.
Wear one patch up to 8 hours. Repeat as necessary, but no more than 3 times daily. Discard patch after single use. Reseal pouch after opening. Children under 12 years of age: Consult a physician.
- Other Information
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
THERA CARE COLD HOT MEDICATED PATCH
menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71101-954 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5 g in 100 g Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE (UNII: 05JZI7B19X) DIHYDROXYALUMINUM AMINOACETATE (UNII: DO250MG0W6) GLYCERIN (UNII: PDC6A3C0OX) KAOLIN (UNII: 24H4NWX5CO) METHYLPARABEN (UNII: A2I8C7HI9T) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLPARABEN (UNII: Z8IX2SC1OH) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) POLYVINYL ALCOHOL (100000 MW) (UNII: 949E52Z6MY) ALCOHOL (UNII: 3K9958V90M) ACRYLIC ACID/DIMETHICONE METHACRYLATE/ETHYLHEXYL ACRYLATE COPOLYMER (UNII: E5024Y9FFQ) EDETATE DISODIUM (UNII: 7FLD91C86K) SORBITOL (UNII: 506T60A25R) PEG-6 SORBITAN OLEATE (UNII: 58O7V09UCI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71101-954-05 5 in 1 BOX 10/01/2020 1 9 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 10/01/2020 Labeler - Veridian Healthcare (830437997) Establishment Name Address ID/FEI Business Operations Unexo Life Sciences, Private Limited 872260479 manufacture(71101-954)