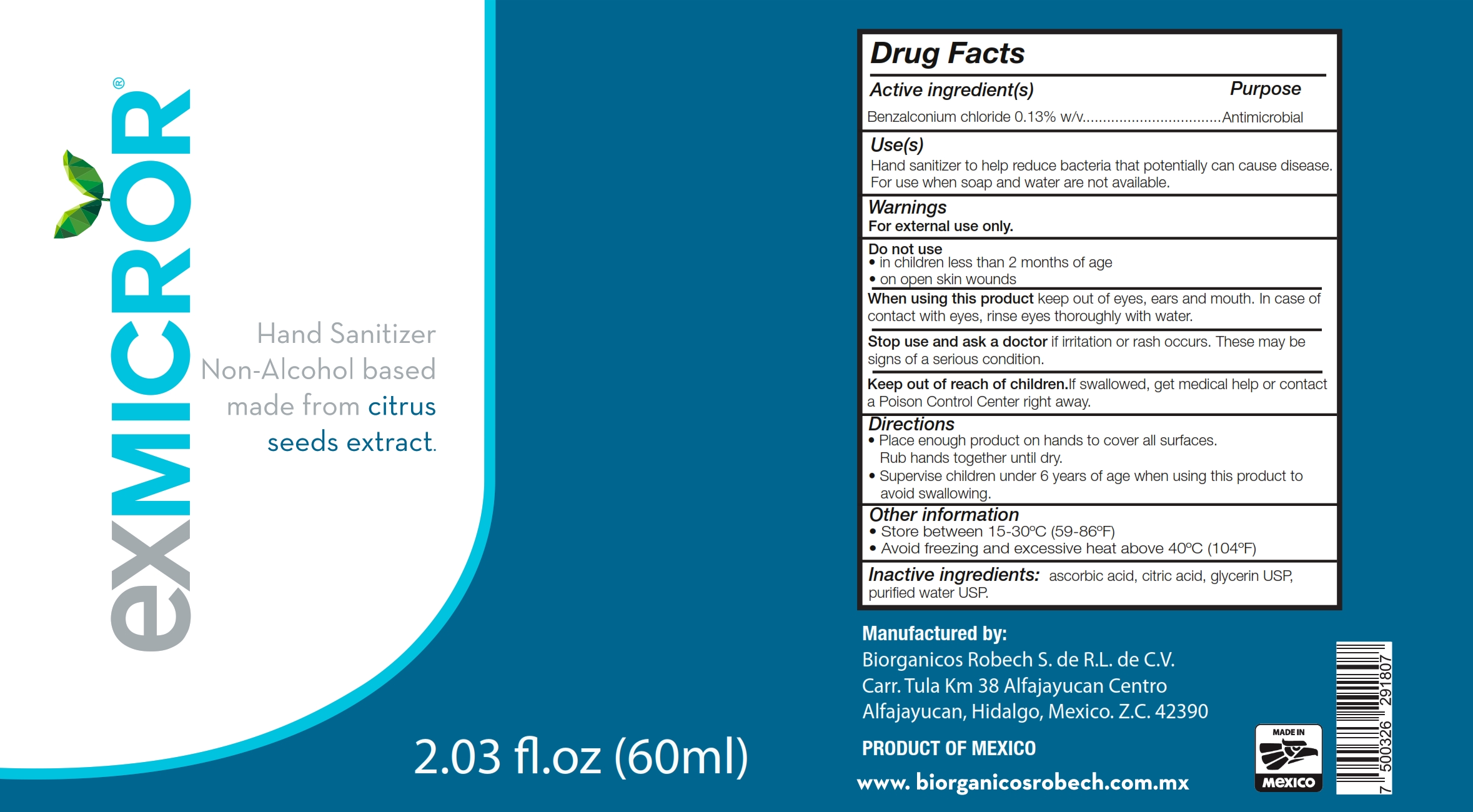
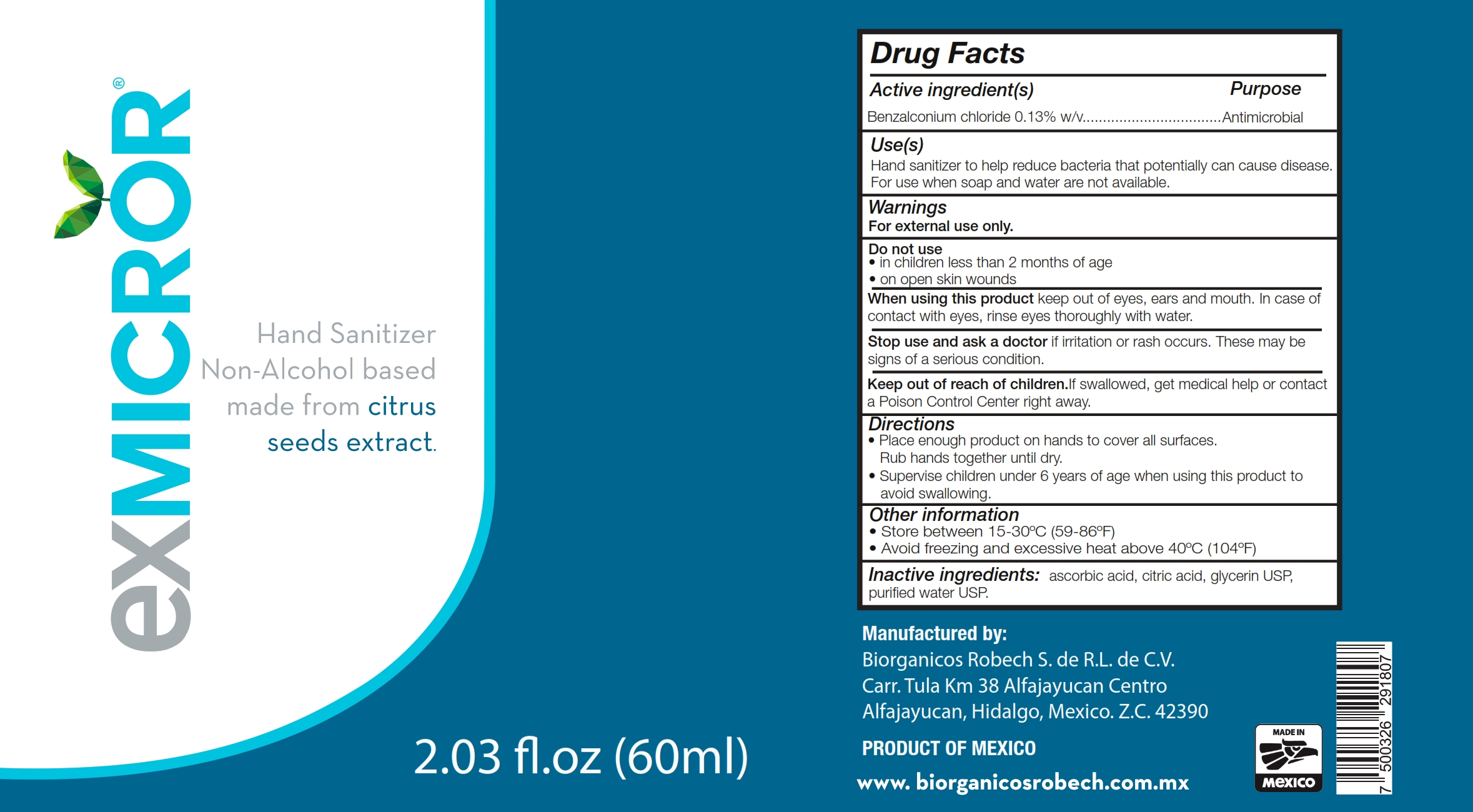
Label: EXMICROR- benzalkonium chloride solution
-
NDC Code(s):
77733-002-03,
77733-002-04,
77733-002-05,
77733-002-06, view more77733-002-07, 77733-002-08
- Packager: Biorganicos Robech, S. de R.L. de C.V.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- EXMICROR 60ml - HAND SANITIZER
- EXMICROR 125ml - HAND SANITIZER
- EXMICROR 250ml - HAND SANITIZER
- EXMICROR 500ml - HAND SANITIZER
- EXMICROR 1000ml - HAND SANITIZER
- EXMICROR 5000ml - HAND SANITIZER
-
INGREDIENTS AND APPEARANCE
EXMICROR
benzalkonium chloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:77733-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) ASCORBIC ACID (UNII: PQ6CK8PD0R) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:77733-002-03 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/24/2022 2 NDC:77733-002-04 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/24/2022 3 NDC:77733-002-05 125 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/24/2022 4 NDC:77733-002-06 250 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/24/2022 5 NDC:77733-002-07 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/24/2022 6 NDC:77733-002-08 5000 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/24/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/24/2022 Labeler - Biorganicos Robech, S. de R.L. de C.V. (951577331) Registrant - Biorganicos Robech, S. de R.L. de C.V. (951577331) Establishment Name Address ID/FEI Business Operations Biorganicos Robech, S. de R.L. de C.V. 951577331 manufacture(77733-002) , label(77733-002)