Label: VVARDIS SET STRONG MINT- sodium monofluorophosphate, sodium fluoride kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 79763-008-01 - Packager: Swiss Shine Beauty AG
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 19, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
Shake well before use.
Adults and children 6 years of age and older: Use once a day after brushing your teeth with a toothpaste.
Vigorously swish 10 milliliters of rinse between your teeth for 1 minute and then spit out.
Do not swallow the rinse.
Do not eat or drink for 30 minutes after rinsing.
Instruct children under 12 years of age in good rinsing habits (to minimize swallowing).
Supervise children as necessary until capable of using without supervision.
Children under 6 years of age: Consult a dentist or doctor. -
DOSAGE & ADMINISTRATION
Directions
Adults and children 2 years of age and older: Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor.
Instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing)
Supervise children as necessary until capable of using without supervison.
Children under 2 years of age: consult a dentist or doctor. - INACTIVE INGREDIENT
-
INACTIVE INGREDIENT
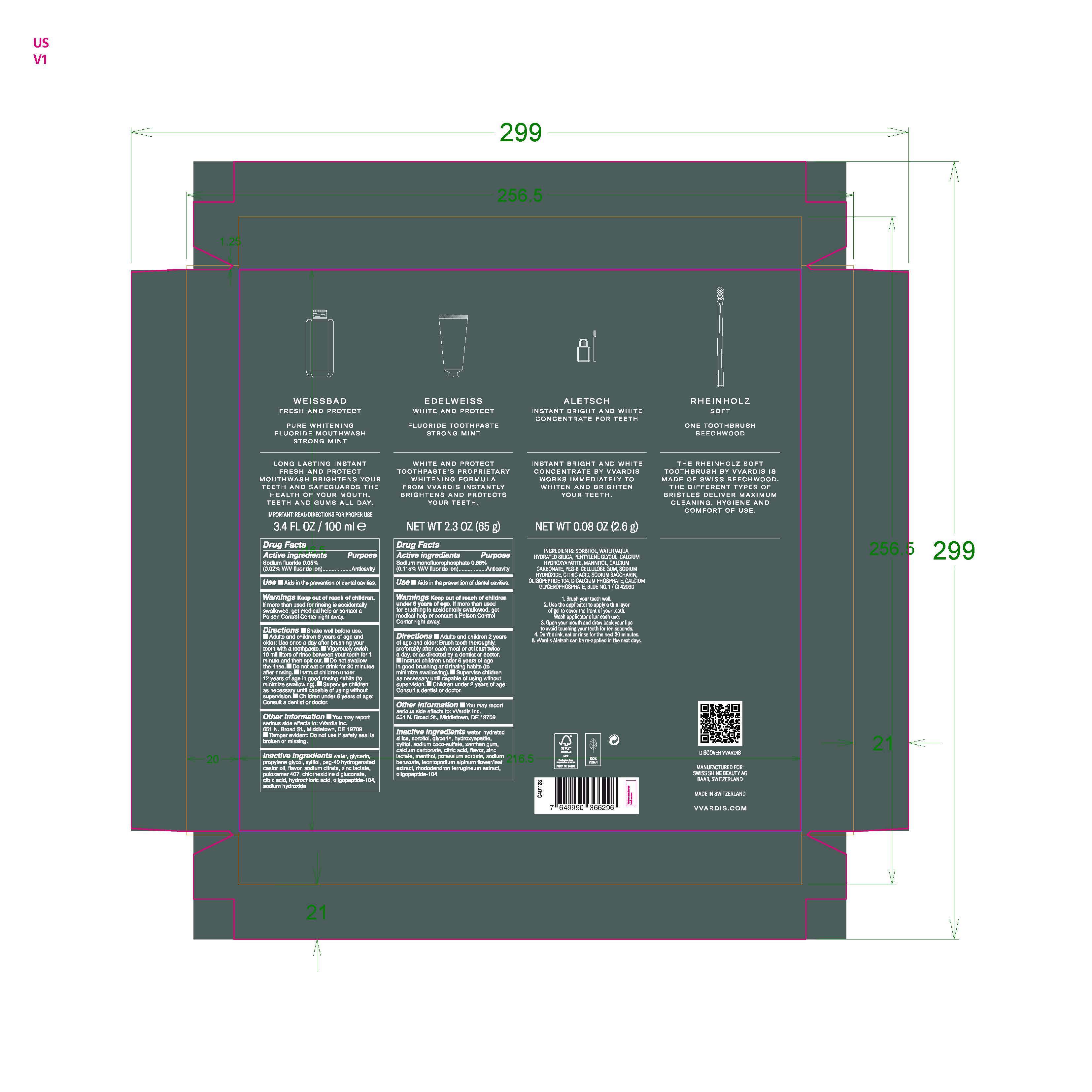
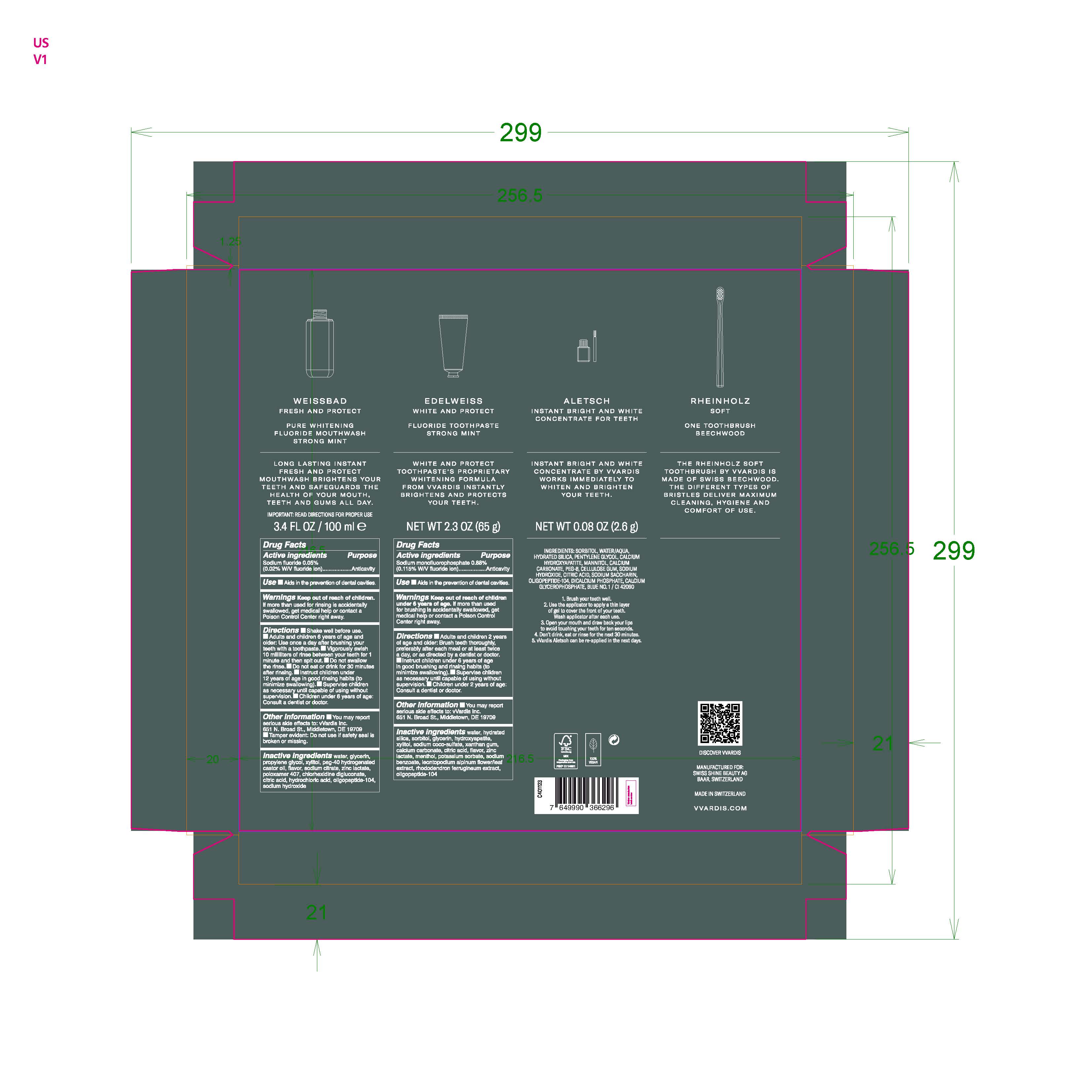
Inactive Ingredients water, hydrated silica, sorbitol, glycerin, hydroxyapatite, xylitol, sodium coco-sulfate, xanthan gum, calcium carbonate, citric acid, flavor, zinc lactate, menthol, potassium sorbate, sodium benzoate, leontopodium alpinum flower/leaf extract, rhododendron ferrugineum extract, oligopeptide-104
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VVARDIS SET STRONG MINT
sodium monofluorophosphate, sodium fluoride kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79763-008 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79763-008-01 1 in 1 KIT 10/14/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 100 mL in 100 Part 2 1 TUBE 65 g in 65 Part 3 1 BOTTLE, WITH APPLICATOR 2.6 g in 100 Part 1 of 3 PURE WHITENING FLUORIDE MOUTHWASH STRONG MINT
sodium fluoride liquidProduct Information Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) POLOXAMER 407 (UNII: TUF2IVW3M2) XYLITOL (UNII: VCQ006KQ1E) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) HYDROCHLORIC ACID (UNII: QTT17582CB) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM HYDROXIDE (UNII: 55X04QC32I) ZINC LACTATE (UNII: 2GXR25858Y) SODIUM CITRATE (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 100 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 10/15/2020 Part 2 of 3 FLUORIDE STRONG MINT
sodium monofluorophosphate gelProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.88 g in 100 g Inactive Ingredients Ingredient Name Strength SODIUM COCO-SULFATE (UNII: 3599J29ANH) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) GLYCERIN (UNII: PDC6A3C0OX) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ZINC LACTATE (UNII: 2GXR25858Y) CALCIUM CARBONATE (UNII: H0G9379FGK) RHODODENDRON FERRUGINEUM WHOLE (UNII: FO6B1S7BZU) LEONTOPODIUM ALPINUM FLOWERING TOP (UNII: QQC1AK06RK) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) WATER (UNII: 059QF0KO0R) HYDRATED SILICA (UNII: Y6O7T4G8P9) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) XYLITOL (UNII: VCQ006KQ1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 65 g in 1 TUBE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 10/15/2020 Part 3 of 3 INSTANT BRIGHT AND WHITE CONCENTRATE FOR TEETH
other oral hygiene products gelProduct Information Route of Administration DENTAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR SORBITOL (UNII: 506T60A25R) INGR HYDRATED SILICA (UNII: Y6O7T4G8P9) INGR WATER (UNII: 059QF0KO0R) INGR MANNITOL (UNII: 3OWL53L36A) INGR PENTYLENE GLYCOL (UNII: 50C1307PZG) INGR POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) INGR TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) INGR CALCIUM CARBONATE (UNII: H0G9379FGK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2.6 g in 1 BOTTLE, WITH APPLICATOR; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 10/15/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part356 10/14/2020 Labeler - Swiss Shine Beauty AG (480422958) Establishment Name Address ID/FEI Business Operations System Kosmetik Produktionsgesellschaft fur kosmetische Erzeugnisse mbH 328825757 manufacture(79763-008) Establishment Name Address ID/FEI Business Operations Marvinpac SA 482060329 pack(79763-008)