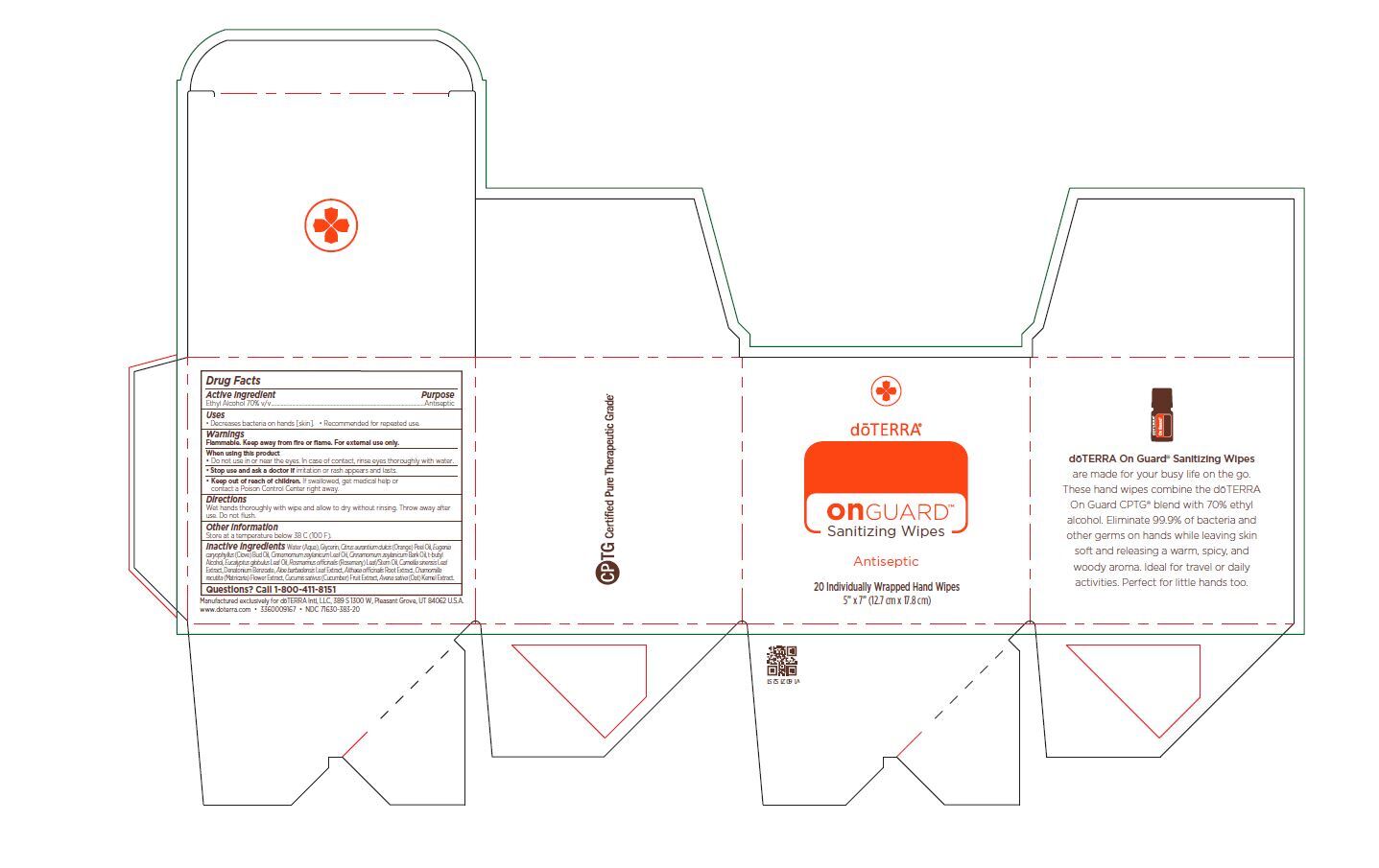
Label: ON GUARD SANITIZING WIPES- alcohol cloth
- NDC Code(s): 71630-383-11, 71630-383-20
- Packager: doTERRA International, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
-
Inactive ingredients
Water (Aqua), Glycerin, Citrus aurantium dulcis (Orange) Peel Oil, Eugenia caryophyllus (Clove) Bud Oil, Cinnamomum zeylanicum Leaf Oil, Cinnamomum zeylanicum Bark Oil, t-butyl
Alcohol, Eucalyptus globulus Leaf Oil, Rosmarinus officinalis (Rosemary) Leaf/Stem Oil, Camellia sinensis Leaf
Extract, Denatonium Benzoate, Aloe barbadensis Leaf Extract, Althaea officinalis Root Extract, Chamomilla recutita (Matricaria) Flower Extract, Cucumis sativus (Cucumber) Fruit Extract, Avena sativa (Oat) Kernel Extract. - Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ON GUARD SANITIZING WIPES
alcohol clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71630-383 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength AVENA SATIVA WHOLE (UNII: 5P8D0Z74RG) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) MATRICARIA RECUTITA FLOWERING TOP (UNII: 3VNC7T6Z02) EUCALYPTUS OIL (UNII: 2R04ONI662) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) CINNAMON LEAF OIL (UNII: S92U8SQ71V) ROSEMARY OIL (UNII: 8LGU7VM393) CLOVE OIL (UNII: 578389D6D0) CINNAMON BARK OIL (UNII: XE54U569EC) ORANGE OIL (UNII: AKN3KSD11B) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) ALTHAEA OFFICINALIS ROOT (UNII: TRW2FUF47H) CUCUMIS SATIVUS WHOLE (UNII: 50560UL2YV) GREEN TEA LEAF (UNII: W2ZU1RY8B0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71630-383-20 1400 mL in 1 CARTON; Type 0: Not a Combination Product 10/30/2020 2 NDC:71630-383-11 70 mL in 1 PACKET; Type 0: Not a Combination Product 10/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 10/30/2020 Labeler - doTERRA International, LLC (832274935)