Label: SODIUM CHLORIDE SOLUTION-
- NHRIC Code(s): 50190-141-23
- Packager: PharmaCaribe
- Category: MEDICAL DEVICE
Drug Label Information
Updated November 20, 2013
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
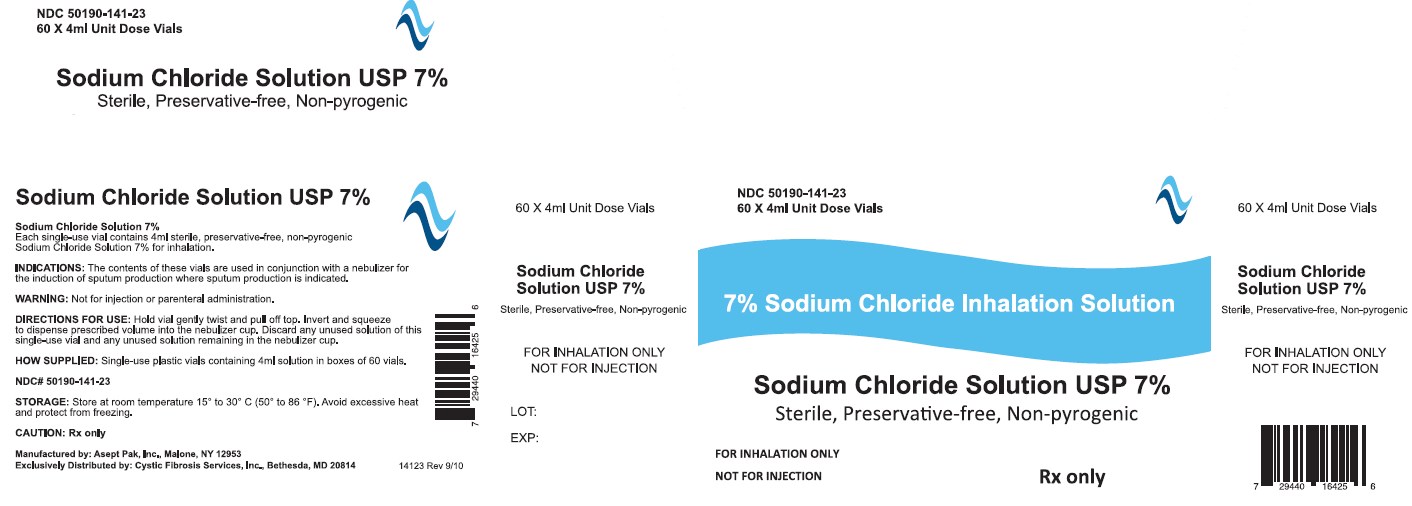
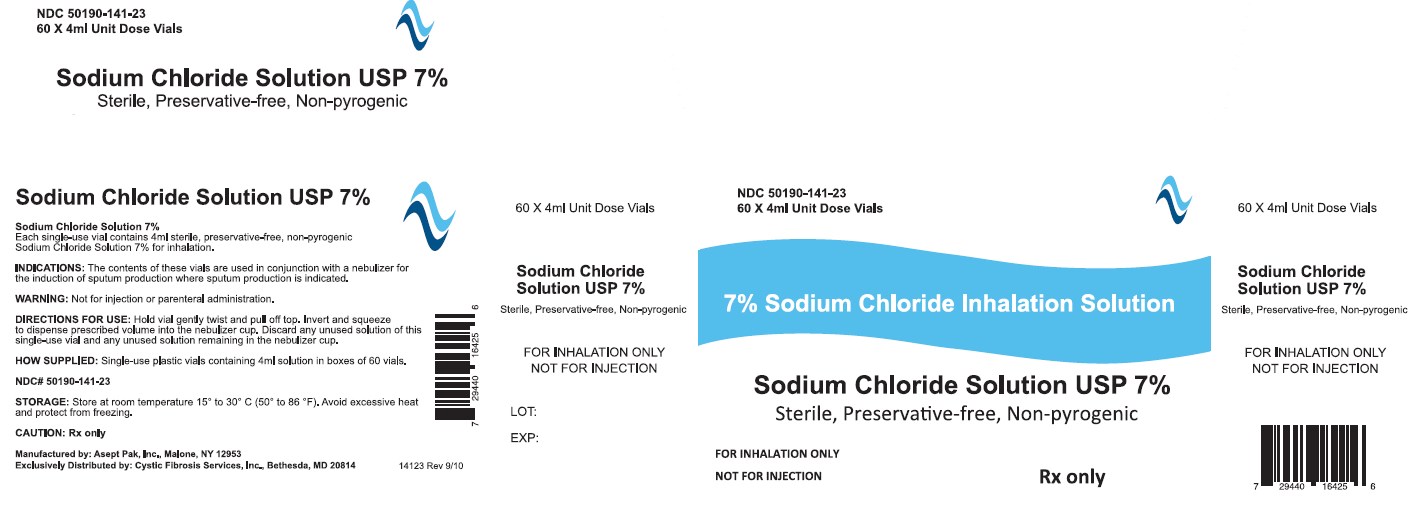
Sodium Chloride Inhalation Solution USP 7% 4 mL
For Respiratory Therapy
Not for parenteral administration.
4 mL, 60 Vials Sterile Unit-Dose Vial
Discard any unused portion of the contents of this single-use vial as well as any unused solution remaining in the nebulizer cup.
Internal contents sterile. External surface of vial not sterile.
- INDICATIONS AND USAGE
- INSTRUCTIONS FOR USE SECTION
- How Supplied
- WARNINGS
- STORAGE
- Sodium Chloride Inhalation Solution USP 7% 4 mL
-
INGREDIENTS AND APPEARANCE
SODIUM CHLORIDE SOLUTION
nebulizer (direct patient interface)Product Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:50190-141 Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698, SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 280 mg in 4 mL Product Characteristics number of times usable 1 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:50190-141-23 60 in 1 BOX 1 4 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date exempt device CAF 08/17/2010 Labeler - PharmaCaribe (011702541) Registrant - PharmaCaribe (011702541) Establishment Name Address ID/FEI Business Operations PharmaCaribe 011702541 label