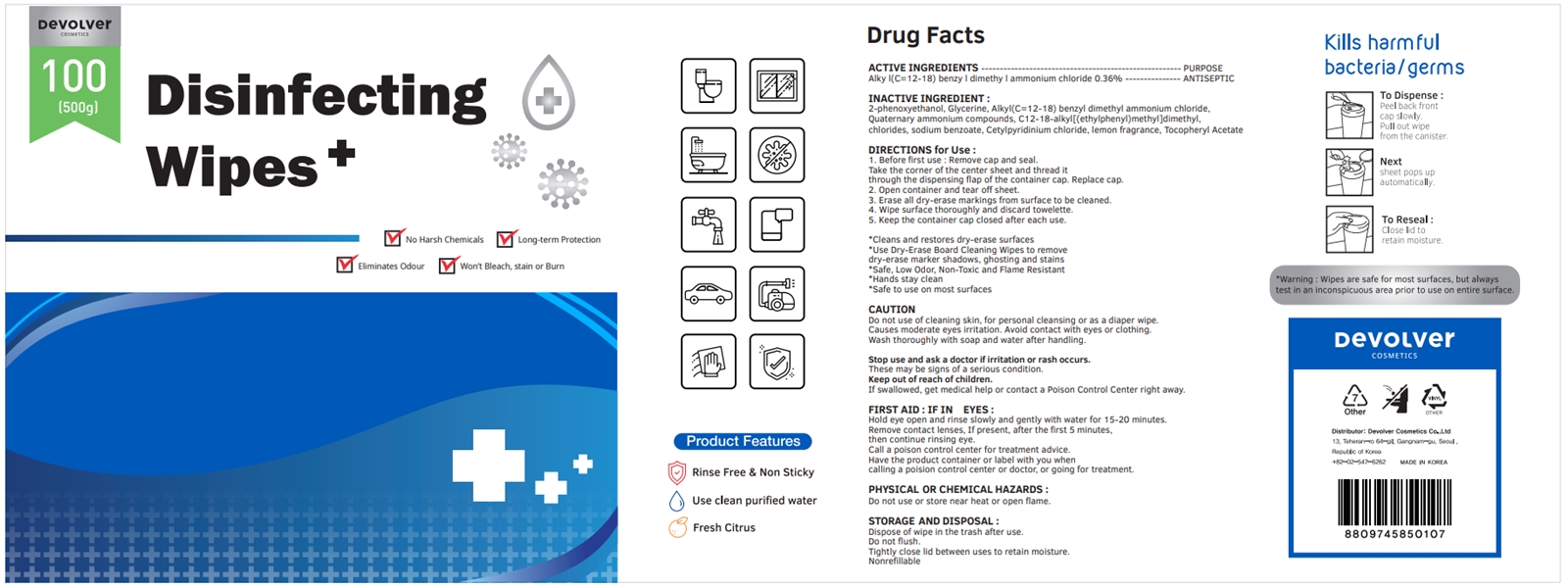
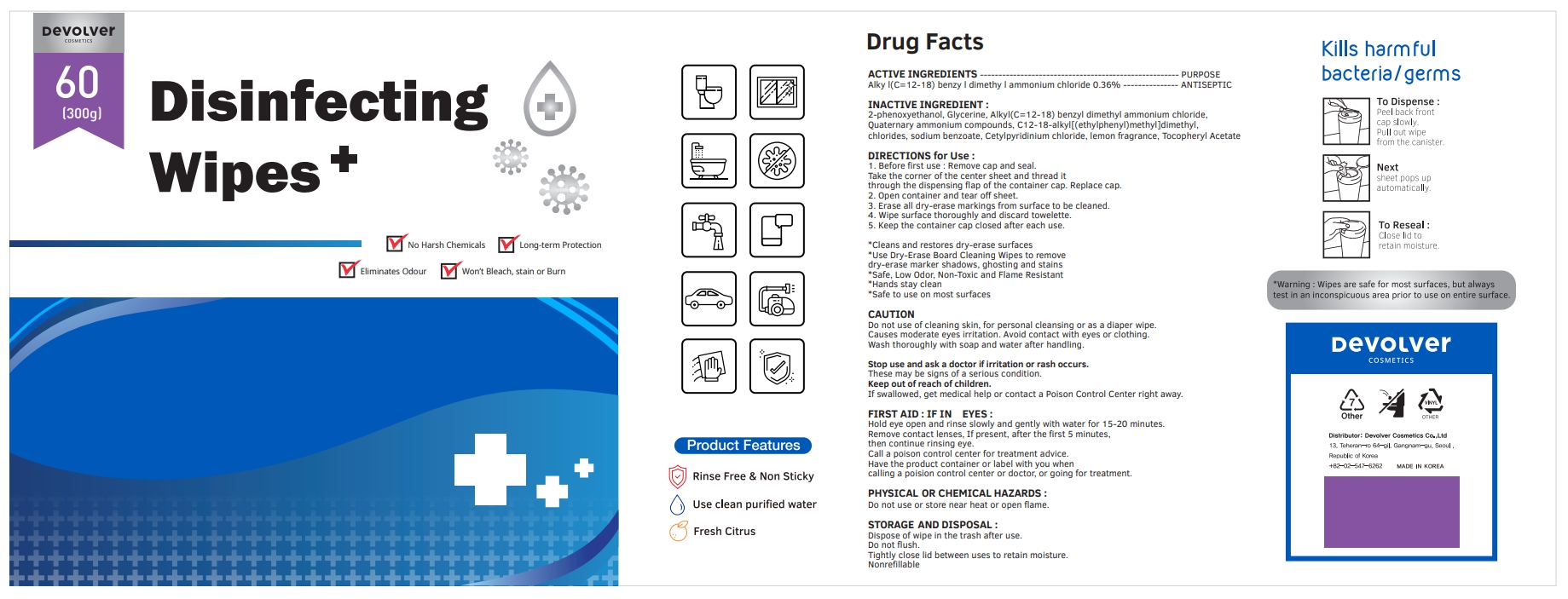
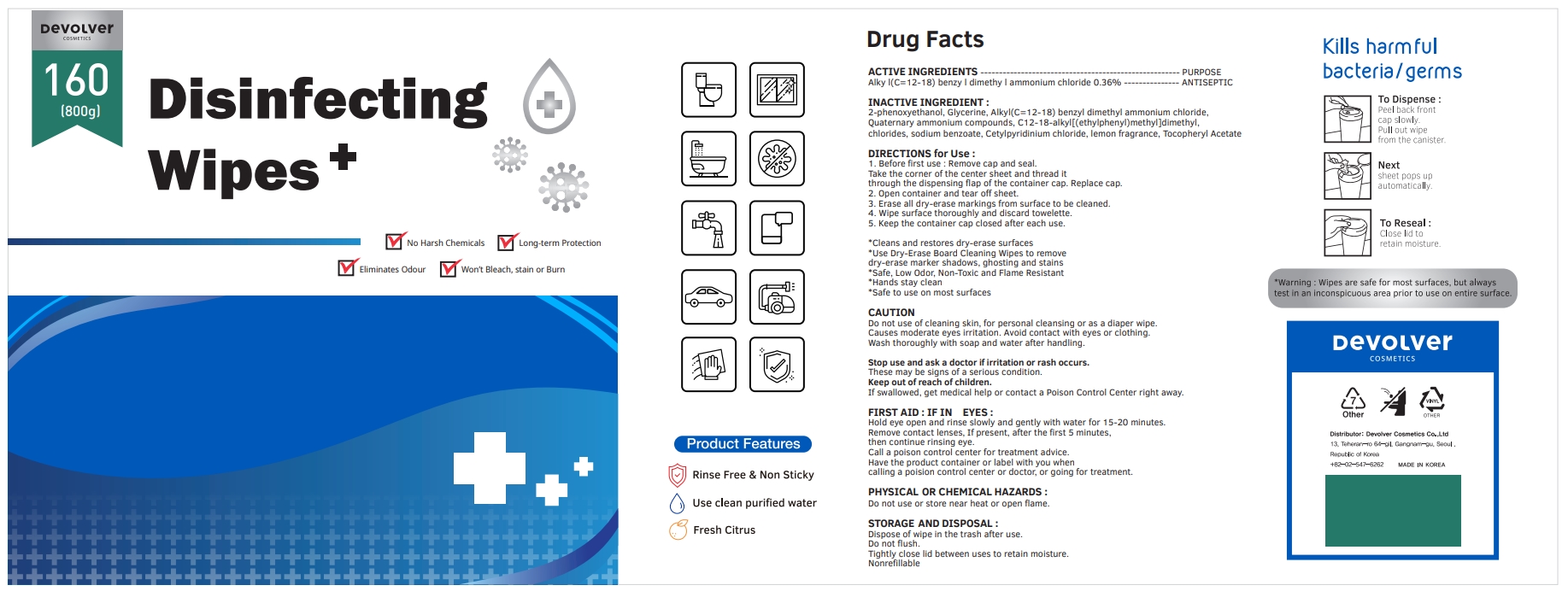
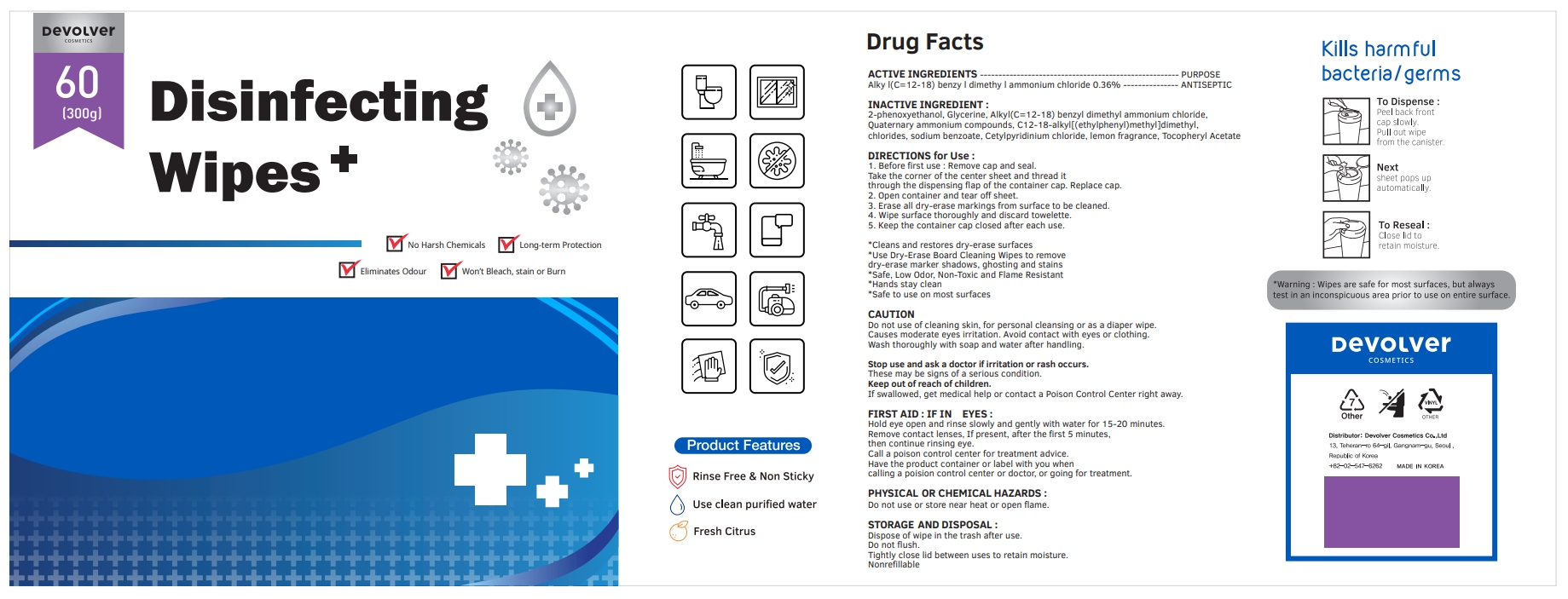
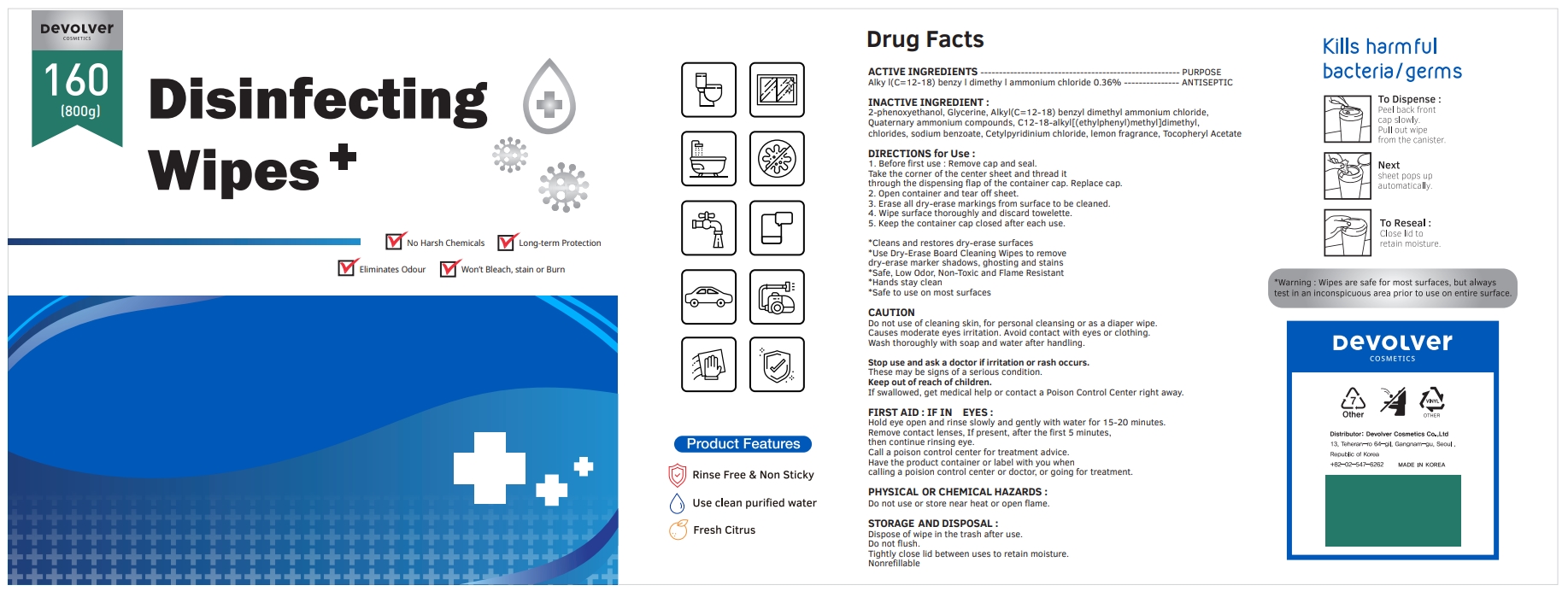
Label: DEVOLVER DISINFECTING WIPES (alkyl- c12-18 benzyl dimethyl ammonium chloride liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 75001-206-01, 75001-206-02, 75001-206-03, 75001-206-04, view more75001-206-05 - Packager: Devolver Cosmetics Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 5, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- PURPOSE
- INACTIVE INGREDIENT
-
DIRECTIONS for Use :
1. Before first use : Remove cap and seal. Take the corner of the center sheet and thread it through the dispensing flap of the container cap. Replace cap.
2. Open container and tear off sheet.
3. Erase all dry-erase markings from surface to be cleaned.
4. Wipe surface thoroughly and discard towelette.
5. Keep the container cap closed after each use.
- DIRECTIONS for Use :
- Warning :
- CAUTION
- CAUTION
- CAUTION
-
FIRST AID : IF IN EYES :
Hold eye open and rinse slowly and gently with water for 15-20 minutes.
Remove contact lenses, If present, after the first 5 minutes, then continue rinsing eye.
Call a poison control center for treatment advice.
Have the product container or label with you when calling a poision control center or doctor, or going for treatment.
- PHYSICAL OR CHEMICAL HAZARDS :
- STORAGE AND DISPOSAL :
- PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
DEVOLVER DISINFECTING WIPES
alkyl (c12-18) benzyl dimethyl ammonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75001-206 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength N-ALKYL DIMETHYL BENZYL AMMONIUM CHLORIDE (C12-C18) (UNII: 9U1Q4T4ZYS) (N-ALKYL DIMETHYL BENZYL AMMONIUM ION (C12-C18) - UNII:3QBE2Z8KR2) N-ALKYL DIMETHYL BENZYL AMMONIUM CHLORIDE (C12-C18) 0.36 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) N-DIALKYL METHYL BENZYL AMMONIUM CHLORIDE (C12-C18) (UNII: 00MFZ4H95Z) SODIUM BENZOATE (UNII: OJ245FE5EU) CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) FRAGRANCE LEMON ORC2001060 (UNII: K1725A7G95) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75001-206-01 500 g in 1 BOTTLE; Type 0: Not a Combination Product 10/08/2020 2 NDC:75001-206-02 300 g in 1 BOTTLE; Type 0: Not a Combination Product 10/08/2020 3 NDC:75001-206-03 400 g in 1 BOTTLE; Type 0: Not a Combination Product 10/08/2020 4 NDC:75001-206-04 600 g in 1 BOTTLE; Type 0: Not a Combination Product 10/08/2020 5 NDC:75001-206-05 800 g in 1 BOTTLE; Type 0: Not a Combination Product 10/08/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/08/2020 Labeler - Devolver Cosmetics Co., Ltd (695733244) Registrant - Devolver Cosmetics Co., Ltd (695733244) Establishment Name Address ID/FEI Business Operations Devolver Cosmetics Co., Ltd 695733244 manufacture(75001-206)