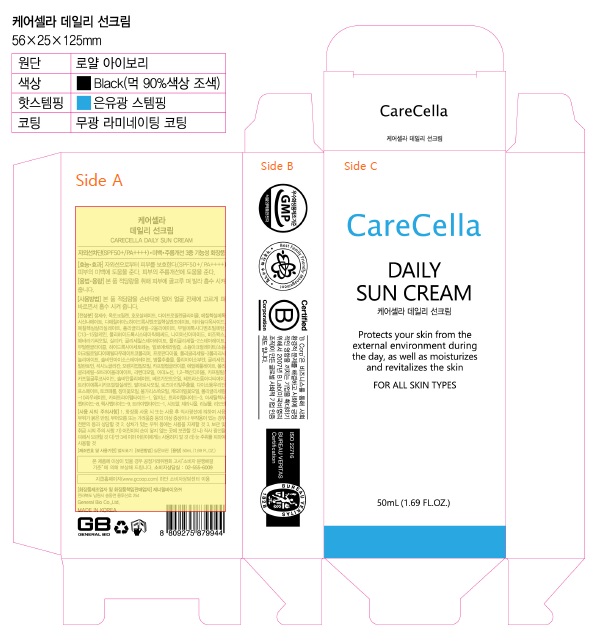
Label: CARECELLA DAILY SUN CREAM- avobenzone, homosalate, octocrylene, octinoxate, octisalate, titanium dioxide cream
- NDC Code(s): 69422-1004-1, 69422-1004-2
- Packager: General Bio Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Avobenzone (2.97%) ------------------------------------------------------------------------------- Sunscreen
Homosalate (7.5%) --------------------------------------------------------------------------------- Sunscreen
Octocrylene (7.5%) --------------------------------------------------------------------------------- Sunscreen
Octyl Methoxycinnamate (7.0%) ---------------------------------------------------------------- Sunscreen
Octyl Salicylate (5.0%) ---------------------------------------------------------------------------- Sunscreen
Titanium Dioxide (5.3%) -------------------------------------------------------------------------- Sunscreen
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
- Apply liberally onto the cleansed entire face and neck 15 minutes prior to sun exposure.
- Reapply as needed or after towel drying, swimming, or sweating.
- Reapply at least every 2 hours: “Sun Protection Measures." Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m. - 2 p.m.; and wear long-sleeved shirts, pants, hats, and sunglasses.
- Children under 6 months: Ask a doctor.
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
1,2-Hexanediol, Caprylyl/Capryl Oligoglucoside, Acetyl Hexapeptide-8, Adenosine, Allantoin, Aloe Vera (Aloe Barbadensis) Leaf Juice, Arginine, Artemisia Vulgaris Top Oil, Bergamot Oil, Butylene Glycol, C13-15 Alkane, Caprylyl/Capryl Oligoglucoside, Caprylyl Glycol, Centella Asiatica Extract, Chamaemelum Nobile (Anthemis Nobilis) Flower Oil, Diethylamino Hydroxybenzoyl Hexyl Benzoate, Dipropylene Glycol, Edetate Sodium, Ethyl Ferulate, Glycerin, Glyceryl Monostearate, Hexapeptide-9, Hydroxyacetophenone, Lavender Oil, Niacinamide, Octinoxate, Octisalate, Orange (Citrus Aurantium Dulcis) Peel Oil Palmarosa Oil, Polyisobutylene, Polyglyceryl-10 Laurate, Polyglyceryl-2 Oleate, Polyglyceryl-2 Stearate, Polyglyceryl-3 Polyricinoleate, Polyglyceryl-5 Trioleate, Polyhydroxystearic Acid, Polyisobutylene, Prezatide, Prezatide Copper, Propanediol, Rosa Rugosa Flower Bud Oil, Rosemary (Rosmarinus Officinalis) Leaf Extract, Silicon Dioxide, Sodium Acrylate/Sodium Acryloyldimethyltaurate Copolymer, Sorbitan Isostearate, Sorbitan Monooleate, Succinoglycan, Sunflower (Helianthus Annuus) Seed Oil, Tocopherol, Triethoxycaprylylsilane, Tripeptide-3, Uridine Monophosphate Disodium, Water, Yellow Wax
- Carecella Daily Sun Cream
-
INGREDIENTS AND APPEARANCE
CARECELLA DAILY SUN CREAM
avobenzone, homosalate, octocrylene, octinoxate, octisalate, titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69422-1004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7 g in 100 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 5.3 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.97 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7.5 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7.5 g in 100 g Inactive Ingredients Ingredient Name Strength C13-15 ALKANE (UNII: 114P5I43UJ) DIETHYLAMINO HYDROXYBENZOYL HEXYL BENZOATE (UNII: ANQ870JD20) HEXAPEPTIDE-9 (UNII: L25414IE41) LAVENDER OIL (UNII: ZBP1YXW0H8) NIACINAMIDE (UNII: 25X51I8RD4) PREZATIDE COPPER (UNII: 6BJQ43T1I9) ROSA RUGOSA FLOWER BUD (UNII: TZ0BE8I3MW) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) SUNFLOWER OIL (UNII: 3W1JG795YI) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHAMAEMELUM NOBILE FLOWER OIL (UNII: UB27587839) EDETATE SODIUM (UNII: MP1J8420LU) CENTELLA ASIATICA (UNII: 7M867G6T1U) BERGAMOT OIL (UNII: 39W1PKE3JI) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL/CAPRYL OLIGOGLUCOSIDE (UNII: E00JL9G9K0) DIPROPYLENE GLYCOL (UNII: E107L85C40) ETHYL FERULATE (UNII: 5B8915UELW) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) CITRUS AURANTIUM FRUIT RIND (UNII: 055456JHI7) POLYGLYCERYL-2 OLEATE (UNII: 5759J47SAM) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) PROPANEDIOL (UNII: 5965N8W85T) ALLANTOIN (UNII: 344S277G0Z) ARGININE (UNII: 94ZLA3W45F) POLYGLYCERYL-5 TRIOLEATE (UNII: SZ1T9D7ZKX) ADENOSINE (UNII: K72T3FS567) ROSEMARY (UNII: IJ67X351P9) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) ALOE VERA LEAF (UNII: ZY81Z83H0X) ARTEMISIA VULGARIS TOP OIL (UNII: 72Q967Y48V) YELLOW WAX (UNII: 2ZA36H0S2V) POLYGLYCERYL-2 STEARATE (UNII: 253MC0P0YV) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) PREZATIDE (UNII: 39TG2H631E) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) URIDINE MONOPHOSPHATE DISODIUM (UNII: KD8E20071T) WATER (UNII: 059QF0KO0R) PALMAROSA OIL (UNII: 0J3G3O53ST) POLYGLYCERYL-10 LAURATE (UNII: MPJ2Q8WI8G) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69422-1004-2 1 in 1 BOX 10/07/2020 1 NDC:69422-1004-1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/07/2020 Labeler - General Bio Co., Ltd. (695273863) Registrant - General Bio Co., Ltd. (695273863) Establishment Name Address ID/FEI Business Operations General Bio Co., Ltd. 695273863 manufacture(69422-1004)