Label: BESIVANCE- besifloxacin suspension
- NDC Code(s): 50090-1241-0
- Packager: A-S Medication Solutions
- This is a repackaged label.
- Source NDC Code(s): 24208-446
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BESIVANCE safely and effectively. See full prescribing information for BESIVANCE.
BESIVANCE® (besifloxacin ophthalmic suspension), for topical ophthalmic use
Initial U.S. Approval: 2009INDICATIONS AND USAGE
BESIVANCE® (besifloxacin ophthalmic suspension) 0.6% is a quinolone antimicrobial indicated for the treatment of bacterial conjunctivitis caused by susceptible isolates of the following bacteria:
Aerococcus viridans*, CDC coryneform group G, Corynebacterium pseudodiphtheriticum*, Corynebacterium striatum*, Haemophilus influenzae, Moraxella catarrhalis*, Moraxella lacunata*, Pseudomonas aeruginosa*, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus hominis*, Staphylococcus lugdunensis*, Staphylococcus warneri*, Streptococcus mitis group, Streptococcus oralis, Streptococcus pneumoniae, Streptococcus salivarius*
*Efficacy for this organism was studied in fewer than 10 infections. (1)
DOSAGE AND ADMINISTRATION
Instill one drop in the affected eye(s) 3 times a day, 4 to 12 hours apart for 7 days. (2)
DOSAGE FORMS AND STRENGTHS
Ophthalmic suspension: besifloxacin 6 mg/mL (0.6%) (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reaction reported in 2% of patients treated with BESIVANCE was conjunctival redness. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb Incorporated at 1-800-553-5340 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.2 Growth of Resistant Organisms with Prolonged Use
5.3 Avoidance of Contact Lenses
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
BESIVANCE® (besifloxacin ophthalmic suspension) 0.6% is indicated for the treatment of bacterial conjunctivitis caused by susceptible isolates of the following bacteria:
Aerococcus viridans*
CDC coryneform group G
Corynebacterium pseudodiphtheriticum*
Corynebacterium striatum*
Haemophilus influenzae
Moraxella catarrhalis*
Moraxella lacunata*
Pseudomonas aeruginosa*
Staphylococcus aureus
Staphylococcus epidermidis
Staphylococcus hominis*
Staphylococcus lugdunensis*
Staphylococcus warneri*
Streptococcus mitis group
Streptococcus oralis
Streptococcus pneumoniae
Streptococcus salivarius*
*Efficacy for this organism was studied in fewer than 10 infections.
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.2 Growth of Resistant Organisms with Prolonged Use
As with other anti-infectives, prolonged use of BESIVANCE (besifloxacin ophthalmic suspension) 0.6% may result in overgrowth of non-susceptible organisms, including fungi. If super-infection occurs, discontinue use and institute alternative therapy. Whenever clinical judgment dictates, the patient should be examined with the aid of magnification, such as slit-lamp biomicroscopy, and, where appropriate, fluorescein staining.
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described below reflect exposure to BESIVANCE in approximately 1,000 patients between 1 and 98 years old with clinical signs and symptoms of bacterial conjunctivitis.
The most frequently reported ocular adverse reaction was conjunctival redness, reported in approximately 2% of patients.
Other adverse reactions reported in patients receiving BESIVANCE occurring in approximately 1-2% of patients included: blurred vision, eye pain, eye irritation, eye pruritus and headache.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available human data for the use of BESIVANCE during pregnancy to inform any drug-associated risks; however, systemic exposure to besifloxacin from ocular administration is low [see Clinical Pharmacology (12.3)].Oral administration of besifloxacin to pregnant rats during organogenesis or during the prenatal and postnatal period did not produce adverse embryofetal or offspring effects at clinically relevant systemic exposures [see Data].
Data
Animal DataIn an embryofetal development study in rats, the administration of besifloxacin at oral doses up to 1,000 mg/kg/day during organogenesis was not associated with visceral or skeletal malformations in rat fetuses, although this dose was associated with maternal toxicity (reduced body weight gain and food consumption) and maternal mortality. Increased post-implantation loss, decreased fetal body weights, and decreased fetal ossification were also observed. At this dose, the mean Cmax in the rat dams was approximately 20 mcg/mL, approximately 46,500 times the mean plasma concentrations measured in humans at the recommended human ophthalmic dose (RHOD). The No Observed Adverse Effect Level (NOAEL) for this embryofetal development study was 100 mg/kg/day (Cmax, 5 mcg/mL, approximately 11,600 times the mean plasma concentrations measured in humans at the RHOD).
In a prenatal and postnatal development study in rats, the NOAELs for both fetal/neonate and maternal toxicity were 100 mg/kg/day. At 1,000 mg/kg/day, pups weighed significantly less than controls and had a reduced neonatal survival rate. Attainment of developmental landmarks and sexual maturation was delayed, although surviving pups from this dose group that were reared to maturity did not demonstrate deficits in behavior, including activity, learning and memory, and their reproductive capacity appeared normal.
8.2 Lactation
Risk Summary
There are no data on the presence of BESIVANCE in human milk, the effects on the breastfed infant, or the effects on milk production. However, systemic exposure to besifloxacin following topical ocular administration is low [see Clinical Pharmacology (12.3)], and it is not known whether measurable levels of besifloxacin would be present in maternal milk following topical ocular administration.
The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for BESIVANCE, and any potential adverse effects on the breastfed infant from BESIVANCE.
8.4 Pediatric Use
The safety and effectiveness of BESIVANCE in infants below one year of age have not been established. The efficacy of BESIVANCE in treating bacterial conjunctivitis in pediatric patients one year or older has been demonstrated in controlled clinical trials [see Clinical Studies (14)].
There is no evidence that the ophthalmic administration of quinolones has any effect on weight-bearing joints, even though systemic administration of some quinolones has been shown to cause arthropathy in immature animals.
-
11 DESCRIPTION
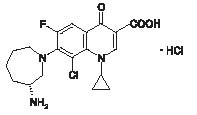
BESIVANCE® (besifloxacin ophthalmic suspension) 0.6% is a sterile ophthalmic suspension of besifloxacin formulated with DuraSite®† (polycarbophil, edetate disodium dihydrate, sodium chloride, sodium hydroxide, and water for injection). Each mL of BESIVANCE contains 6.63 mg besifloxacin hydrochloride equivalent to 6 mg besifloxacin base. It is an 8-chloro fluoroquinolone anti-infective for topical ophthalmic use.
C19H21ClFN3O3•HCl
Molecular Weight 430.30
Chemical Name: (+)-7-[(3R)-3-aminohexahydro-1H-azepin-1-yl]-8-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid hydrochloride.
Besifloxacin hydrochloride is a white to pale yellowish-white powder.
Each mL contains:
Active: besifloxacin 0.6% (6 mg/mL);
Inactives: polycarbophil, mannitol, poloxamer 407, sodium chloride, edetate disodium dihydrate, sodium hydroxide, and water for injection.
Preservative: benzalkonium chloride 0.01%
BESIVANCE is an isotonic suspension with an osmolality of approximately 290 mOsm/kg.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Plasma concentrations of besifloxacin were measured in adult patients with suspected bacterial conjunctivitis who received BESIVANCE bilaterally three times a day (16 doses total). Following the first and last dose, the maximum plasma besifloxacin concentration in each patient was less than 1.3 ng/mL. The mean besifloxacin Cmax was 0.37 ng/mL on Day 1 and 0.43 ng/mL on Day 6. The average elimination half-life of besifloxacin in plasma following multiple dosing was estimated to be 7 hours.
12.4 Microbiology
Besifloxacin is an 8-chloro fluoroquinolone with an N-1 cyclopropyl group. The compound has activity against Gram-positive and Gram-negative bacteria due to the inhibition of both bacterial DNA gyrase and topoisomerase IV. DNA gyrase is an essential enzyme required for replication, transcription and repair of bacterial DNA. Topoisomerase IV is an essential enzyme required for partitioning of the chromosomal DNA during bacterial cell division. Besifloxacin is bactericidal with minimum bactericidal concentrations (MBCs) generally within one dilution of the minimum inhibitory concentrations (MICs).
The mechanism of action of fluoroquinolones, including besifloxacin, is different from that of aminoglycoside, macrolide, and β-lactam antibiotics. Therefore, besifloxacin may be active against pathogens that are resistant to these antibiotics and these antibiotics may be active against pathogens that are resistant to besifloxacin. In vitro studies demonstrated cross-resistance between besifloxacin and some fluoroquinolones.
In vitro resistance to besifloxacin develops via multiple-step mutations and occurs at a general frequency of <3.3 x 10-10 for Staphylococcus aureus and <7 x 10-10 for Streptococcus pneumoniae.
Besifloxacin has been shown to be active against most isolates of the following bacteria both in vitro and in conjunctival infections treated in clinical trials [see Indications and Usage (1)]:
- Aerococcus viridans*
- CDC coryneform group G
- Corynebacterium pseudodiphtheriticum*
- Corynebacterium striatum*
- Haemophilus influenzae
- Moraxella catarrhalis*
- Moraxella lacunata*
- Pseudomonas aeruginosa*
- Staphylococcus aureus
- Staphylococcus epidermidis
- Staphylococcus hominis*
- Staphylococcus lugdunensis*
- Staphylococcus warneri*
- Streptococcus mitis group
- Streptococcus oralis
- Streptococcus pneumoniae
- Streptococcus salivarius*
- *Efficacy for this organism was studied in fewer than 10 infections.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to determine the carcinogenic potential of besifloxacin have not been performed.
No in vitro mutagenic activity of besifloxacin was observed in an Ames test (up to 3.33 mcg/plate) on bacterial tester strains Salmonella typhimurium TA98, TA100, TA1535, TA1537 and Escherichia coli WP2uvrA. However, it was mutagenic in S. typhimurium strain TA102 and E. coli strain WP2 (pKM101). Positive responses in these strains have been observed with other quinolones and are likely related to topoisomerase inhibition.
Besifloxacin induced chromosomal aberrations in CHO cells in vitro and it was positive in an in vivo mouse micronucleus assay at oral doses ≥1,500 mg/kg. Besifloxacin did not induce unscheduled DNA synthesis in hepatocytes cultured from rats given the test compound up to 2,000 mg/kg by the oral route. In a fertility and early embryonic development study in rats, besifloxacin did not impair the fertility of male or female rats at oral doses of up to 500 mg/kg/day. This dose is approximately 26,500 times higher than the mean plasma concentration measured in humans at the recommended human ophthalmic dose.
-
14 CLINICAL STUDIES
In a randomized, double-masked, vehicle-controlled, multicenter clinical trial, in which patients 1-98 years of age were dosed 3 times a day for 5 days, BESIVANCE was superior to its vehicle in patients with bacterial conjunctivitis. Clinical resolution was achieved in 45% (90/198) for the BESIVANCE-treated group versus 33% (63/191) for the vehicle-treated group (difference 12%, 95% CI 3% - 22%). Microbiological outcomes demonstrated a statistically significant eradication rate for causative pathogens of 91% (181/198) for the BESIVANCE-treated group versus 60% (114/191) for the vehicle-treated group (difference 31%, 95% CI 23% - 40%). Microbiologic eradication does not always correlate with clinical outcome in anti-infective trials.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Handling the Container
Advise patients to avoid contaminating the applicator tip with material from the eye, fingers or other source.Use with Contact Lenses
Advise patients not to wear contact lenses if they have signs or symptoms of bacterial conjunctivitis or during the course of therapy with BESIVANCE.Dosing Instructions
Patients should be instructed to invert closed bottle (upside down) and shake once before each use.Distributed by:
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USAManufactured by:
Bausch & Lomb Incorporated
Tampa, FL 33637 USAPatented. See https://patents.bausch.com for US patent information.
BESIVANCE is a trademark of Bausch & Lomb Incorporated or its affiliates.
© 2022 Bausch & Lomb Incorporated or its affiliates
†DuraSite is a trademark of Sun Pharma Global FZE.
9142709 (folded)
9142609 (flat) - Besifloxacin
-
INGREDIENTS AND APPEARANCE
BESIVANCE
besifloxacin suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50090-1241(NDC:24208-446) Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BESIFLOXACIN (UNII: BFE2NBZ7NX) (BESIFLOXACIN - UNII:BFE2NBZ7NX) BESIFLOXACIN 6 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) POLYCARBOPHIL (UNII: W25LM17A4W) MANNITOL (UNII: 3OWL53L36A) POLOXAMER 407 (UNII: TUF2IVW3M2) SODIUM CHLORIDE (UNII: 451W47IQ8X) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50090-1241-0 1 in 1 CARTON 11/28/2014 1 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022308 05/28/2009 Labeler - A-S Medication Solutions (830016429) Establishment Name Address ID/FEI Business Operations A-S Medication Solutions 830016429 RELABEL(50090-1241)