Label: DIANEAL LOW CALCIUM WITH DEXTROSE- sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solution
-
NDC Code(s):
0941-0684-01,
0941-0684-04,
0941-0688-01,
0941-0688-04, view more0941-0692-01, 0941-0692-04
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Health Care Provider Letter
-
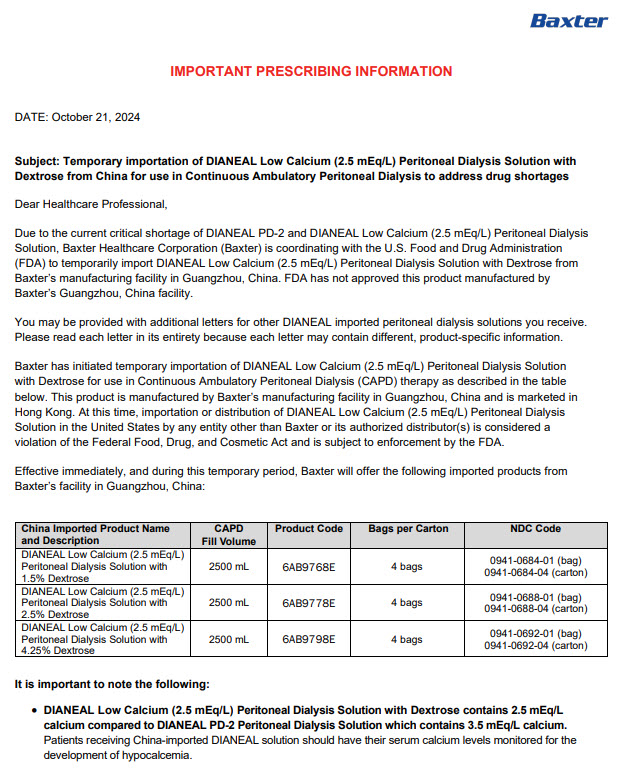
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
6AB9768E 2500ml
(APPROX 90ml EXCESS)
3000ml NOMINAL SIZE CONTAINERBaxterLogo
Dianeal ®Low Calcium(2.5mEq/L)
Peritoneal Dialysis Solution
With 1.5% DextroseEACH 100 ml CONTAINS1.5g DEXTROSE HYDROUS USP
538 mg SODIUM CHLORIDE USP 448 mg SODIUM LACTATE
18.3 mg CALCIUM CHLORIDE USP 5.08 mg MAGNESIUM
CHLORIDE USP pH5.2 (4.5 to 6.5)
mEq/LSODIUM 132 CALCIUM 2.5 MAGNESIUM 0.5
CHLORIDE 95 LACTATE 40
OSMOLARITY344 mOsmol/L(CALC)
STERILE NON PYROGENICPOTASSIUM CHLORIDE TO BE ADDED ONLY UNDER
THE DIRECTION OF A PHYSICIANREAD PACKAGE INSERT FOR FULL INFORMATION
FOR INTRAPERITONEAL ADMINISTRATION ONLY
CAUTIONSSQUEEZE AND INSPECT INNER BAG WHICH
MAINTAINS PRODUCT STERILITY DISCARD IF
LEAKS ARE FOUND
DO NOT USE UNLESS SOLUTION IS CLEAR
DISCARD UNUSED PORTION
STORE UNIT IN MOISTURE BARRIER OVERWRAP AT ROOM
TEMPERATURE (UNDER25°C) UNTIL READY TO USE
AVOID EXCESSIVE HEAT SEE INSERTUltrabag TMCONTAINER PL-146 PLASTIC
MANUFACTURED BY
BAXTER HEALTHCARE (GUANGZHOU) CO LTD
GUANGZHOU CHINA
(AN AFFILIATE OF BAXTER WORLD TRADE INC USA)
HK-62710
DIRECTIONSTO BE USED AS DIRECTED BY THE PHYSICIANPrescription Drug
Manufacturer Address:
Jiaoyuan Road, Dongji Industrial District,
GETDD, Guangzhou, P.R. ChinaLow Calcium 1.5% Dextrose
1.5 LOW CALCIUM WITH 1.5% DEXTROSE
ULTRABAG 2500mlX4
LOT G00000000 EXP JAN 006AB9768E
S/N 0000
6AB9778E 2500ml
(APPROX 90ml EXCESS)
3000ml NOMINAL SIZE CONTAINERBaxterLogo
Dianeal ®Low Calcium(2.5mEq/L)
Peritoneal Dialysis Solution
With 2.5% DextroseEACH 100ml CONTAINS2.5g DEXTROSE HYDROUS USP
538 mg SODIUM CHLORIDE USP 448 mg SODIUM LACTATE
18.3 mg CALCIUM CHLORIDE USP 5.08 mg MAGNESIUM
CHLORIDE USP pH5.2 (4.5 to 6.5)
mEq/LSODIUM 132 CALCIUM 2.5 MAGNESIUM 0.5
CHLORIDE 95 LACTATE 40
OSMOLARITY395 mOsmol/L(CALC)
STERILE NON PYROGENICPOTASSIUM CHLORIDE TO BE ADDED ONLY UNDER
THE DIRECTION OF A PHYSICIANREAD PACKAGE INSERT FOR FULL INFORMATION
FOR INTRAPERITONEAL ADMINISTRATION ONLY
CAUTIONSSQUEEZE AND INSPECT INNER BAG WHICH
MAINTAINS PRODUCT STERILITY DISCARD IF
LEAKS ARE FOUND
DO NOT USE UNLESS SOLUTION IS CLEAR
DISCARD UNUSED PORTION
STORE UNIT IN MOISTURE BARRIER OVERWRAP AT ROOM
TEMPERATURE (UNDER25°C) UNTIL READY TO USE
AVOID EXCESSIVE HEAT SEE INSERTUltrabag TMCONTAINER PL-146 PLASTIC
MANUFACTURED BY
BAXTER HEALTHCARE (GUANGZHOU) CO LTD
GUANGZHOU CHINA
(AN AFFILIATE OF BAXTER WORLD TRADE INC USA)
HK-62709
DIRECTIONSTO BE USED AS DIRECTED BY THE PHYSICIANPrescription Drug
Manufacturer Address:
Jiaoyuan Road, Dongji Industrial District,
GETDD, Guangzhou, P.R. ChinaLow Calcium 2.5% Dextrose
2.5 LOW CALCIUM WITH 2.5% DEXTROSE
ULTRABAG 2500mlX4
LOT G00000000 EXP JAN 006AB9778E
S/N 0000
6AB9798E 2500ml
(APPROX 90ml EXCESS)
3000ml NOMINAL SIZE CONTAINERBaxterLogo
Dianeal ®Low Calcium(2.5mEq/L)
Peritoneal Dialysis Solution
With 4.25% DextroseEACH 100ml CONTAINS4.25g DEXTROSE HYDROUS USP
538 mg SODIUM CHLORIDE USP 448 mg SODIUM LACTATE
18.3 mg CALCIUM CHLORIDE USP 5.08 mg MAGNESIUM
CHLORIDE USP pH5.2 (4.5 to 6.5)
mEq/LSODIUM 132 CALCIUM 2.5 MAGNESIUM 0.5
CHLORIDE 95 LACTATE 40 OSMOLARITY483 mOsmol/L(CALC)
STERILE NON PYROGENICPOTASSIUM CHLORIDE TO BE ADDED ONLY UNDER
THE DIRECTION OF A PHYSICIANWARNINGEXTENSIVE USE OF THIS SOLUTION DURING
ONE PERITONEAL DIALYSIS PROCEDURE CAN RESULT IN
SIGNIFICANT REMOVAL OF WATER FROM THE PATIENT
READ PACKAGE INSERT FOR FULL INFORMATION
FOR INTRAPERITONEAL ADMINISTRATION ONLY
CAUTIONSSQUEEZE AND INSPECT INNER BAG WHICH
MAINTAINS PRODUCT STERILITY DISCARD IF
LEAKS ARE FOUND
DO NOT USE UNLESS SOLUTION IS CLEAR
DISCARD UNUSED PORTION
STORE UNIT IN MOISTURE BARRIER OVERWRAP AT ROOM
TEMPERATURE (UNDER25°C) UNTIL READY TO USE
AVOID EXCESSIVE HEAT SEE INSERTUltrabag TMCONTAINER PL-146 PLASTIC
MANUFACTURED BY
BAXTER HEALTHCARE (GUANGZHOU) CO LTD
GUANGZHOU CHINA
(AN AFFILIATE OF BAXTER WORLD TRADE INC USA)
HK-62711
DIRECTIONSTO BE USED AS DIRECTED BY THE PHYSICIANPrescription Drug
Manufacturer Address:
Jiaoyuan Road, Dongji Industrial District,
GETDD, Guangzhou, P.R. ChinaLow Calcium 4.25% Dextrose
4.25 LOW CALCIUM WITH 4.25% DEXTROSE
ULTRABAG 2500mlX4
LOT G00000000 EXP JAN 006AB9798E
S/N 0000
-
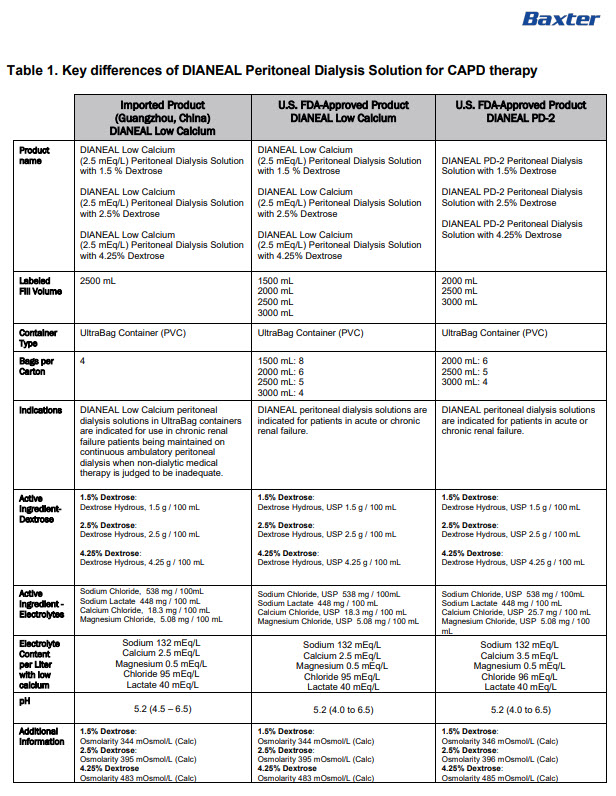
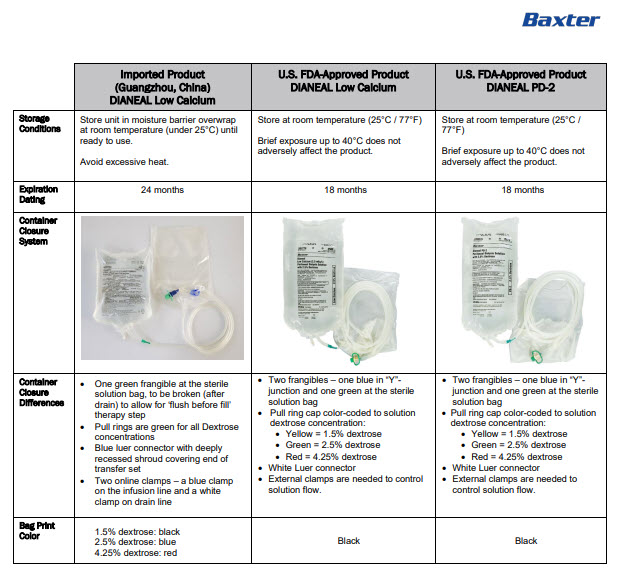
INGREDIENTS AND APPEARANCE
DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0941-0684 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 1.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.3 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0941-0684-04 4 in 1 CARTON 11/01/2024 1 NDC:0941-0684-01 2500 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 11/01/2024 DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0941-0688 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 2.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.3 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0941-0688-04 4 in 1 CARTON 11/01/2024 1 NDC:0941-0688-01 2500 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 11/01/2024 DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0941-0692 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 4.25 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.3 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0941-0692-04 4 in 1 CARTON 11/01/2024 1 NDC:0941-0692-01 2500 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 11/01/2024 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare (Guangzhou) Co., Ltd 421040114 analysis(0941-0684, 0941-0688, 0941-0692) , label(0941-0684, 0941-0688, 0941-0692) , manufacture(0941-0684, 0941-0688, 0941-0692) , pack(0941-0684, 0941-0688, 0941-0692) , sterilize(0941-0684, 0941-0688, 0941-0692)