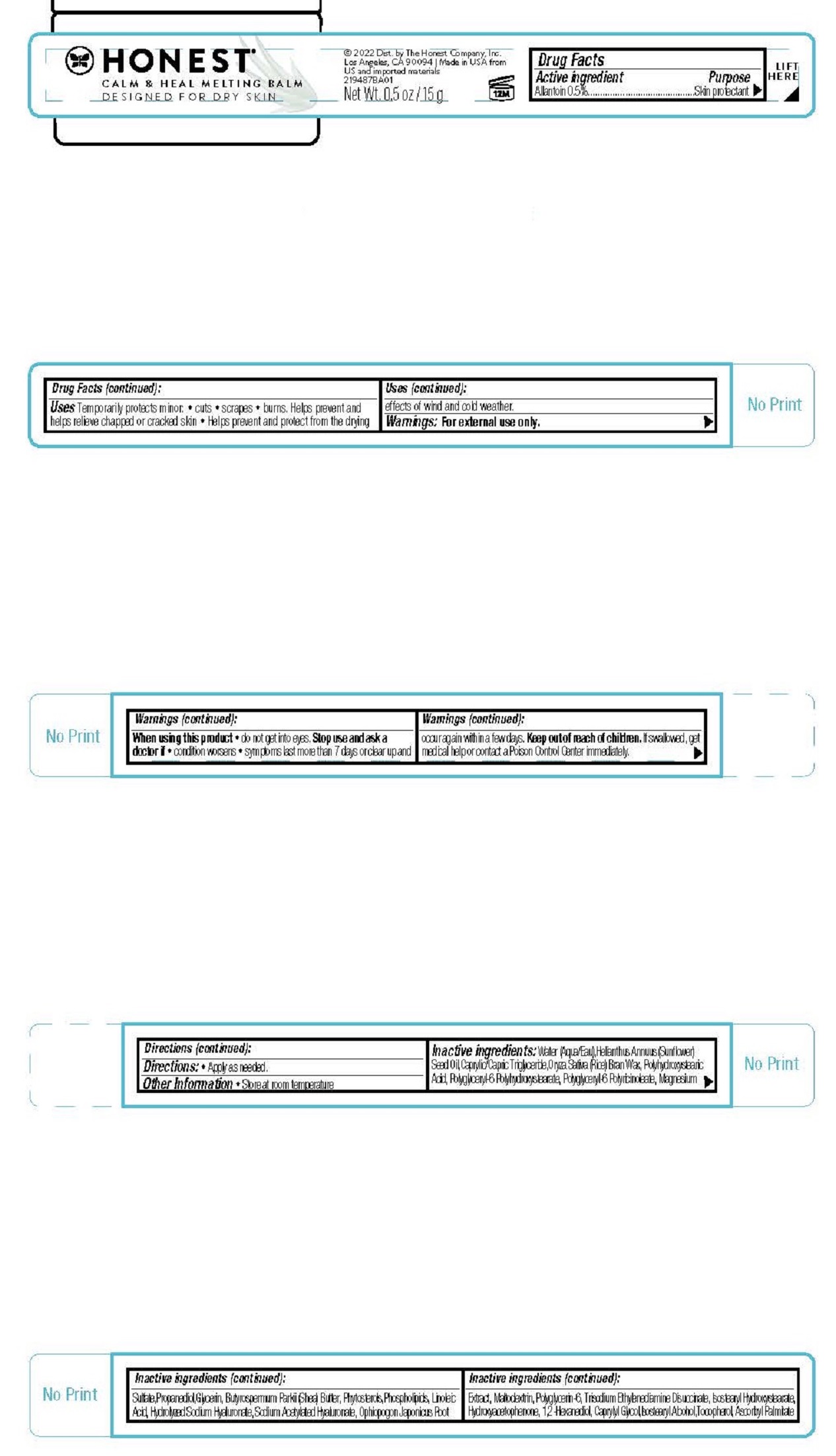
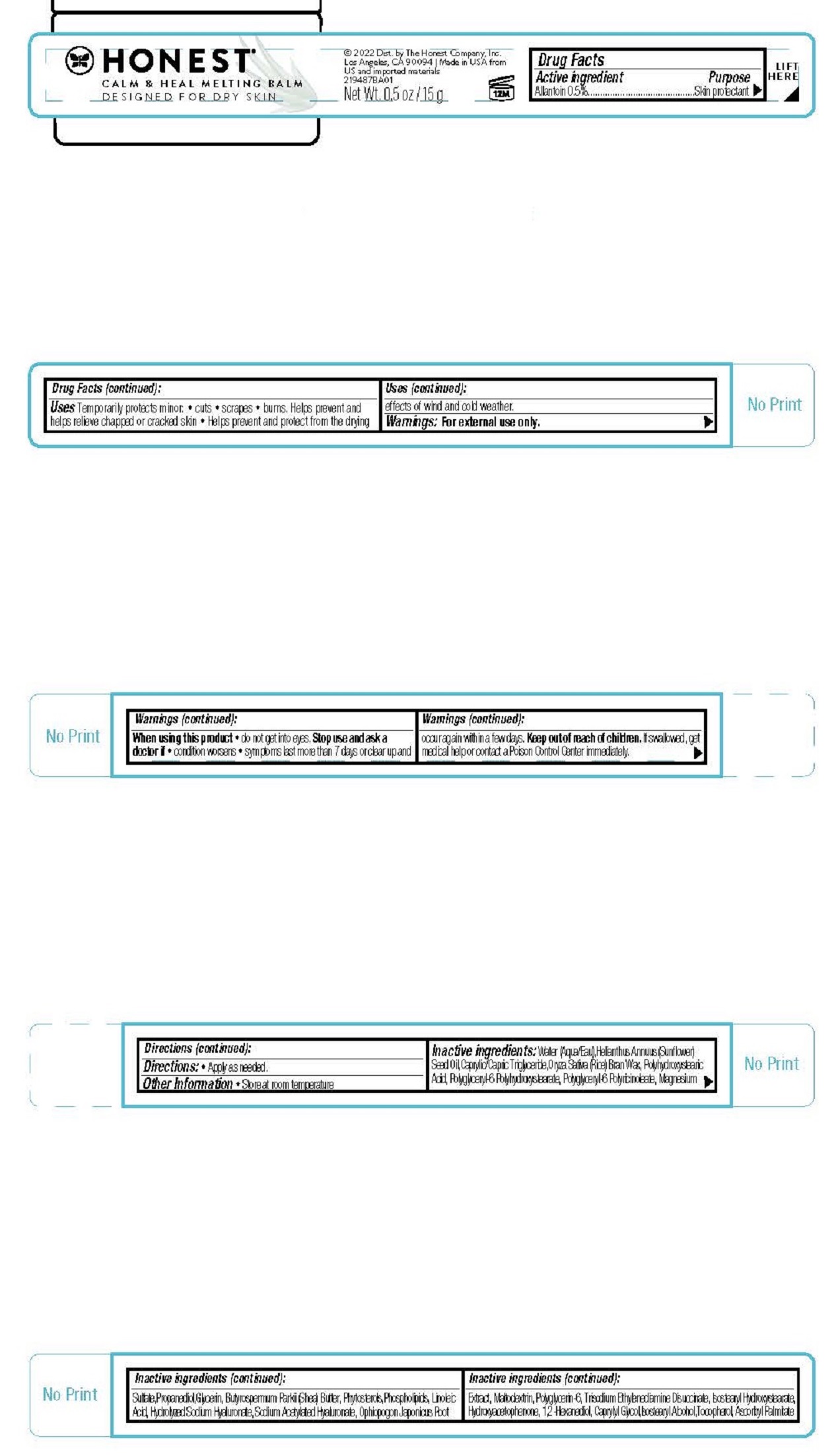
Label: CALM AND HEAL MELTING BALM- allantoin cream
- NDC Code(s): 69366-505-23, 69366-505-28
- Packager: The Honest Company, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- SPL UNCLASSIFIED SECTION
-
INACTIVE INGREDIENT
Inactive ingredients Water (Aqua/Eau),Helianthus Annuus (Sunflower) Seed Oil,Caprylic/Capric Triglyceride,Oryza Sativa (Rice) Bran Wax,Polyhydroxystearic Acid, Polyglyceryl-6 Polyhydroxystearate, Polyglyceryl-6 Polyricinoleate, Magnesium Sulfate,Propanediol,Glycerin, Butyrospermum Parkii (Shea) Butter, Phytosterols,Phospholipids, Linoleic Acid, Hydrolyzed Sodium Hyaluronate, Sodium Acetylated Hyaluronate, Ophiopogon Japonicus Root Extract, Maltodextrin, Polyglycerin-6, Trisodium Ethylenediamine Disuccinate, Isostearyl Hydroxystearate, Hydroxyacetophenone, 1,2-Hexanediol, Caprylyl Glycol,Isostearyl Alcohol,Tocopherol, Ascorbyl Palmitate
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CALM AND HEAL MELTING BALM
allantoin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69366-505 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 0.5 g in 100 g Inactive Ingredients Ingredient Name Strength POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYGLYCERIN-6 (UNII: M51422LRAM) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) WATER (UNII: 059QF0KO0R) SUNFLOWER OIL (UNII: 3W1JG795YI) RICE BRAN (UNII: R60QEP13IC) SOY STEROL (UNII: PL360EPO9J) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) MALTODEXTRIN (UNII: 7CVR7L4A2D) ISOSTEARYL HYDROXYSTEARATE (UNII: F7540880P0) TOCOPHEROL (UNII: R0ZB2556P8) OPHIOPOGON JAPONICUS ROOT (UNII: 90PS6JV9GZ) ASCORBYL PALMITATE (UNII: QN83US2B0N) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) PROPANEDIOL (UNII: 5965N8W85T) GLYCERIN (UNII: PDC6A3C0OX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) LINOLEIC ACID (UNII: 9KJL21T0QJ) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69366-505-23 1 in 1 CARTON 09/29/2020 1 50 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:69366-505-28 1 in 1 CARTON 09/01/2021 2 15 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 09/29/2020 Labeler - The Honest Company, Inc. (969962757)