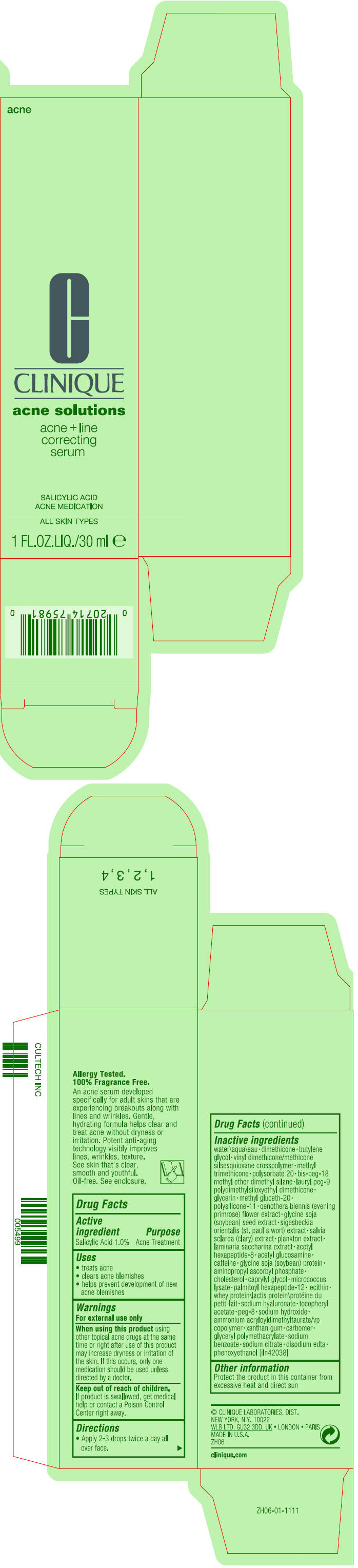
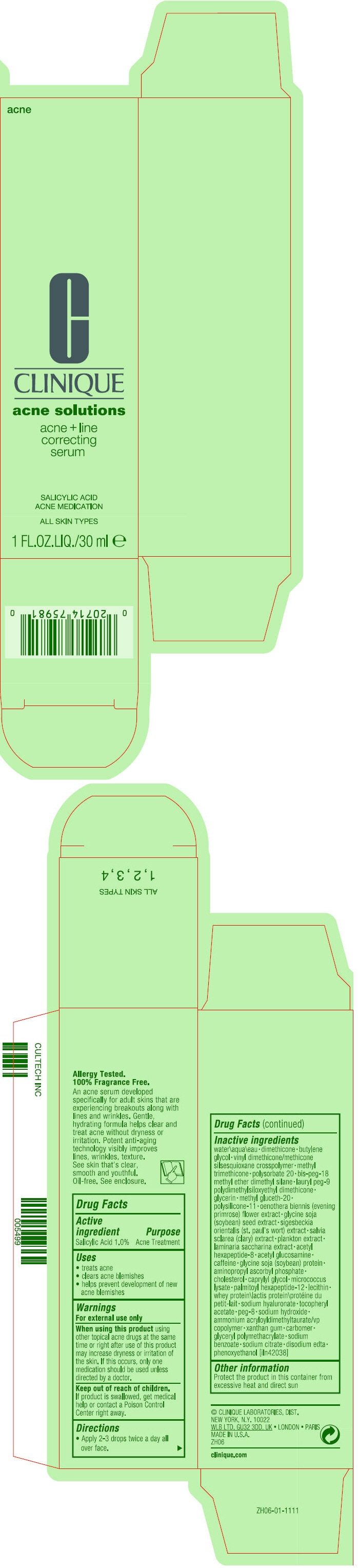
Label: ACNE AND LINE CORRECTING SERUM- salicylic acid lotion
- NDC Code(s): 49527-053-01
- Packager: CLINIQUE LABORATORIES LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
water\aqua\eau • dimethicone • butylene glycol • vinyl dimethicone/methicone silsesquioxane crosspolymer • methyl trimethicone • polysorbate 20 • bis-peg-18 methyl ether dimethyl silane • lauryl peg-9 polydimethylsiloxyethyl dimethicone • glycerin • methyl gluceth-20 • polysilicone-11 • oenothera biennis (evening primrose) flower extract • glycine soja (soybean) seed extract • sigesbeckia orientalis (st. paul's wort) extract • salvia sclarea (clary) extract • plankton extract • laminaria saccharina extract • acetyl hexapeptide-8 • acetyl glucosamine • caffeine • gylcine soja (soybean) protein • aminopropyl ascorbyl phosphate • cholesterol • caprylyl glycol • micrococcus lysate • palmitoyl hexapeptide-12 • lecithin • whey protein\lactis protein\protéine du petit-lait • sodium hyaluronate • tocopheryl acetate • peg-8 • sodium hydroxide • ammonium acryloyldimethyltaurate/vp copolymer • xanthan gum • carbomer • glyceryl polymethacrylate • sodium benzoate • sodium citrate • disodium edta • phenoxyethanol [iln42038]
- Other information
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton
-
INGREDIENTS AND APPEARANCE
ACNE AND LINE CORRECTING SERUM
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49527-053 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) METHYL TRIMETHICONE (UNII: S73ZQI0GXM) POLYSORBATE 20 (UNII: 7T1F30V5YH) BIS-PEG-18 METHYL ETHER DIMETHYL SILANE (UNII: OEB4R3WW9C) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) GLYCERIN (UNII: PDC6A3C0OX) METHYL GLUCETH-20 (UNII: J3QD0LD11P) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) OENOTHERA BIENNIS FLOWER (UNII: Y1YXJ1M6Z5) SOYBEAN (UNII: L7HT8F1ZOD) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) N-ACETYLGLUCOSAMINE (UNII: V956696549) CAFFEINE (UNII: 3G6A5W338E) SOY PROTEIN (UNII: R44IWB3RN5) AMINOPROPYL ASCORBYL PHOSPHATE (UNII: 290O2PQ83R) CHOLESTEROL (UNII: 97C5T2UQ7J) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PALMITOYL HEXAPEPTIDE-12 (UNII: HO4ZT5S86C) WHEY (UNII: 8617Z5FMF6) HYALURONATE SODIUM (UNII: YSE9PPT4TH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SODIUM HYDROXIDE (UNII: 55X04QC32I) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) XANTHAN GUM (UNII: TTV12P4NEE) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49527-053-01 1 in 1 CARTON 08/15/2016 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 08/15/2016 Labeler - CLINIQUE LABORATORIES LLC (044475127) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations The Estee Lauder Inc 802599436 manufacture(49527-053) , pack(49527-053) , label(49527-053)