Label: GABAPENTIN tablet, film coated
-
NDC Code(s):
65841-705-01,
65841-705-05,
65841-705-10,
65841-706-01, view more65841-706-05, 65841-706-10, 65841-706-77
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
- MEDICATION GUIDE
- SPL MEDGUIDE
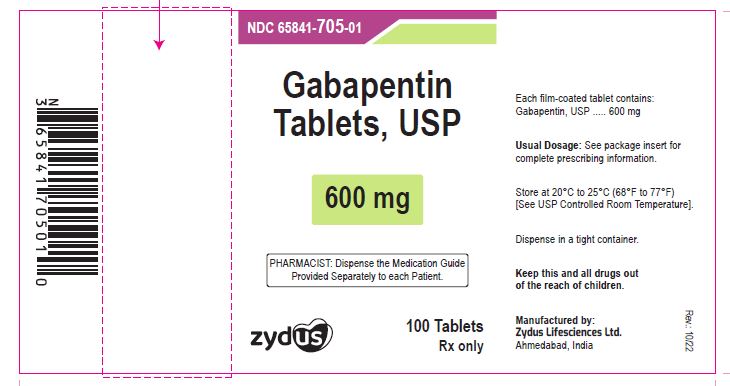
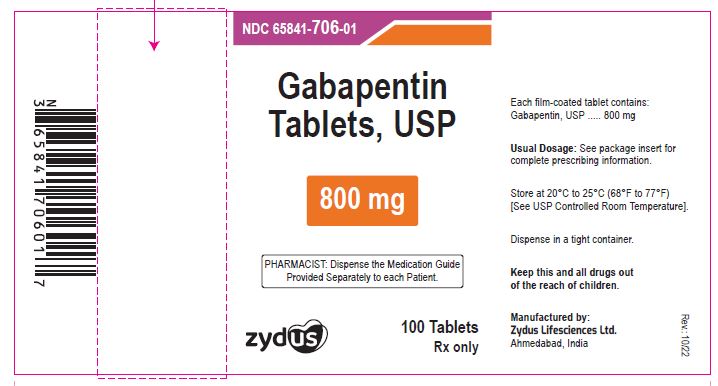
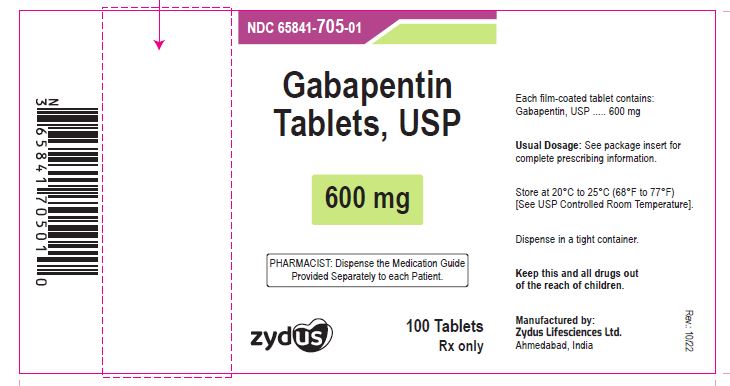
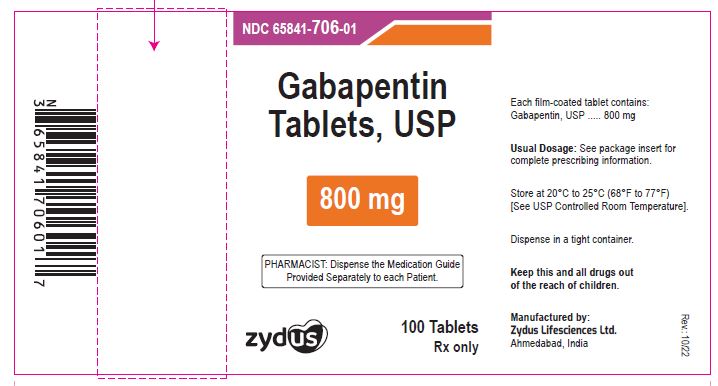
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GABAPENTIN
gabapentin tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-705 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GABAPENTIN (UNII: 6CW7F3G59X) (GABAPENTIN - UNII:6CW7F3G59X) GABAPENTIN 600 mg Inactive Ingredients Ingredient Name Strength COPOVIDONE K25-31 (UNII: D9C330MD8B) HYDROXYPROPYL CELLULOSE, LOW SUBSTITUTED (UNII: 2165RE0K14) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) POLOXAMER 407 (UNII: TUF2IVW3M2) POVIDONE (UNII: FZ989GH94E) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score 2 pieces Shape OVAL (OVAL) Size 17mm Flavor Imprint Code ZE72 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-705-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/16/2012 2 NDC:65841-705-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 10/16/2012 3 NDC:65841-705-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 10/16/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078926 10/16/2012 GABAPENTIN
gabapentin tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-706 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GABAPENTIN (UNII: 6CW7F3G59X) (GABAPENTIN - UNII:6CW7F3G59X) GABAPENTIN 800 mg Inactive Ingredients Ingredient Name Strength COPOVIDONE K25-31 (UNII: D9C330MD8B) HYDROXYPROPYL CELLULOSE, LOW SUBSTITUTED (UNII: 2165RE0K14) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) POLOXAMER 407 (UNII: TUF2IVW3M2) POVIDONE (UNII: FZ989GH94E) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score 2 pieces Shape OVAL (OVAL) Size 20mm Flavor Imprint Code ZE71 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-706-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/16/2012 2 NDC:65841-706-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 10/16/2012 3 NDC:65841-706-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 10/16/2012 4 NDC:65841-706-77 10 in 1 CARTON 10/16/2012 4 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078926 10/16/2012 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 918596198 ANALYSIS(65841-705, 65841-706) , MANUFACTURE(65841-705, 65841-706)