Label: JNN-II CLEAN TOK HAND GEL- alcohol gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 78424-020-01 - Packager: JOY LIFE Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 24, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL
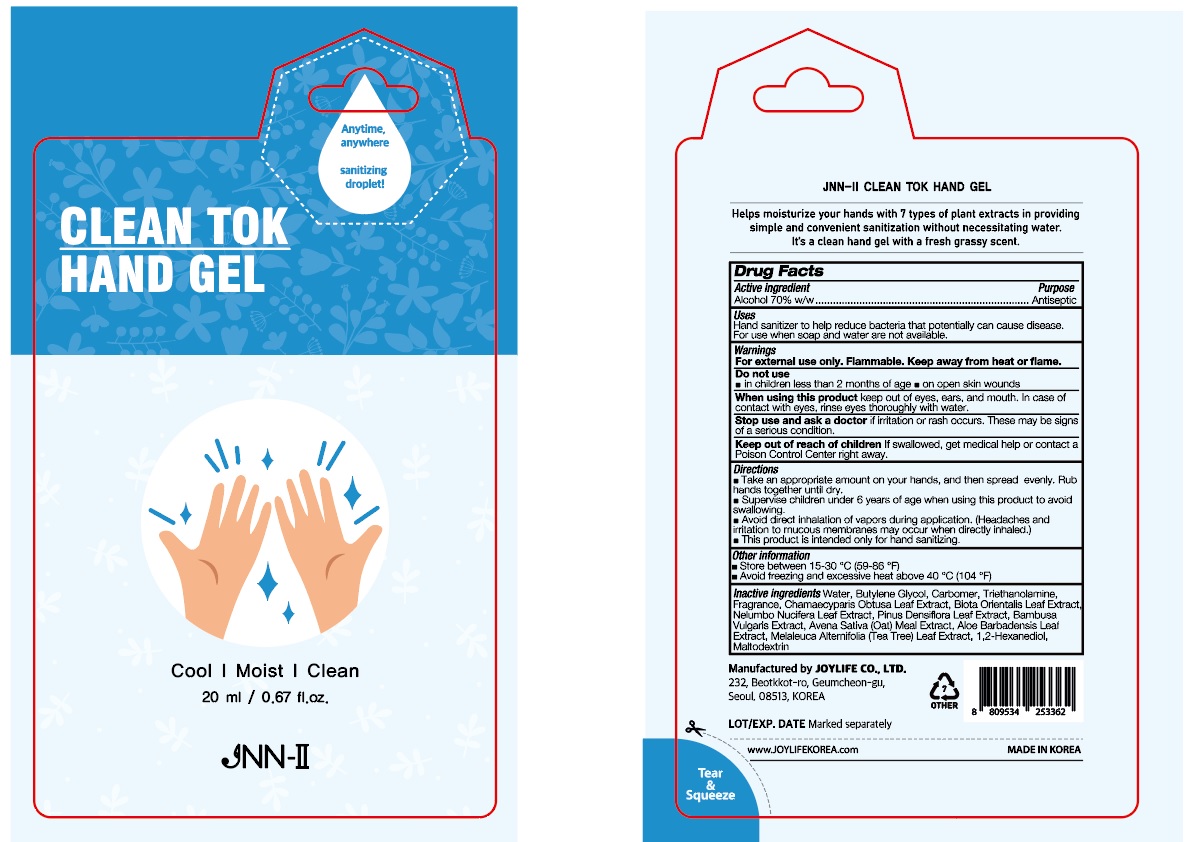
When using this product
keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
■ Take an appropriate amount on your hands, and then spread evenly. Rub hands together until dry.
■ Supervise children under 6 years of age when using this product to avoid swallowing.
■ Avoid direct inhalation of vapors during application. (Headaches and irritation to mucous membranes may occur when directly inhaled.)
■ This product is intended only for hand sanitizing.
Other information
■ Store between 15-30 °C (59-86 °F)
■ Avoid freezing and excessive heat above 40 °C (104 °F)
Inactive ingredients
Water, Butylene Glycol, Carbomer, Triethanolamine, Fragrance, Chamaecyparis Obtusa Leaf Extract, Biota Orientalis Leaf Extract, Nelumbo Nucifera Leaf Extract, Pinus Densiflora Leaf Extract, Bambusa Vulgaris Extract, Avena Sativa (Oat) Meal Extract, Aloe Barbadensis Leaf Extract, Melaleuca Alternifolia (Tea Tree) Leaf Extract, 1,2-Hexanediol, Maltodextrin
-
INGREDIENTS AND APPEARANCE
JNN-II CLEAN TOK HAND GEL
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:78424-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 14 mL in 20 mL Inactive Ingredients Ingredient Name Strength NELUMBO NUCIFERA LEAF (UNII: 60C608DPVT) MALTODEXTRIN (UNII: 7CVR7L4A2D) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) TROLAMINE (UNII: 9O3K93S3TK) CHAMAECYPARIS OBTUSA LEAF (UNII: 7OL154J5XB) BAMBUSA VULGARIS TOP (UNII: FIW80T6P6V) ALOE VERA LEAF (UNII: ZY81Z83H0X) MELALEUCA ALTERNIFOLIA LEAF (UNII: G43C57162K) WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PLATYCLADUS ORIENTALIS LEAF (UNII: 32E5V7G32B) PINUS DENSIFLORA LEAF (UNII: Q1Q9P50WIY) OATMEAL (UNII: 8PI54V663Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78424-020-01 20 mL in 1 POUCH; Type 0: Not a Combination Product 09/23/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/23/2020 Labeler - JOY LIFE Co., Ltd. (689846233) Establishment Name Address ID/FEI Business Operations JOY LIFE Co., Ltd. 689846233 manufacture(78424-020)

