Label: REVLON COLORSTAY LIGHT COVER FOUNDATION- homosalate, octisalate, octocrylene, titanium dioxide,zinc oxide emulsion
- NDC Code(s): 10967-663-01
- Packager: Revlon
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Active Ingredients
- Directions
-
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures, including: Limit time in the sun, especially from 10am-2pm, wear long-sleeved shirts, pants, hats and sunglasses.
-
INACTIVE INGREDIENT
Isododecane, Aqua/Water/Eau, Trimethylsiloxysilicate, Titanium Dioxide, Homosalate,
Zinc Oxide [nano], Dimethicone, Cyclopentasiloxane, Glycerin, Propanediol, Ethylhexyl Salicylate,
PEG-10 Dimethicone, Dimethicone/Vinyl Dimethicone Crosspolymer, Octocrylene, Niacinamide,
PEG/PPG-18/18 Dimethicone, Alumina, Methyl Methacrylate Crosspolymer, Punica Granatum Sterols,
Phenyl Trimethicone, 1,2-Hexanediol, Alcohol Denat., Caffeine, Caprylyl Glycol, Dimethicone
Crosspolymer, Dimethiconol, Disteardimonium Hectorite, Hydrogen Dimethicone, Isoceteth-10,
Lecithin, Magnesium Sulfate, Methicone, Mica, Polyglyceryl-3 Diisostearate, Polysilicone-11,
Propylene Carbonate, Silica, Silica silylate, Tetrasodium EDTA, Tocopheryl Acetate,
Triethoxycaprylylsilane, Iron Oxides (CI 77491), Iron Oxides (CI 77492), Iron Oxides (CI 77499), Titanium Dioxide (CI 77891). - DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
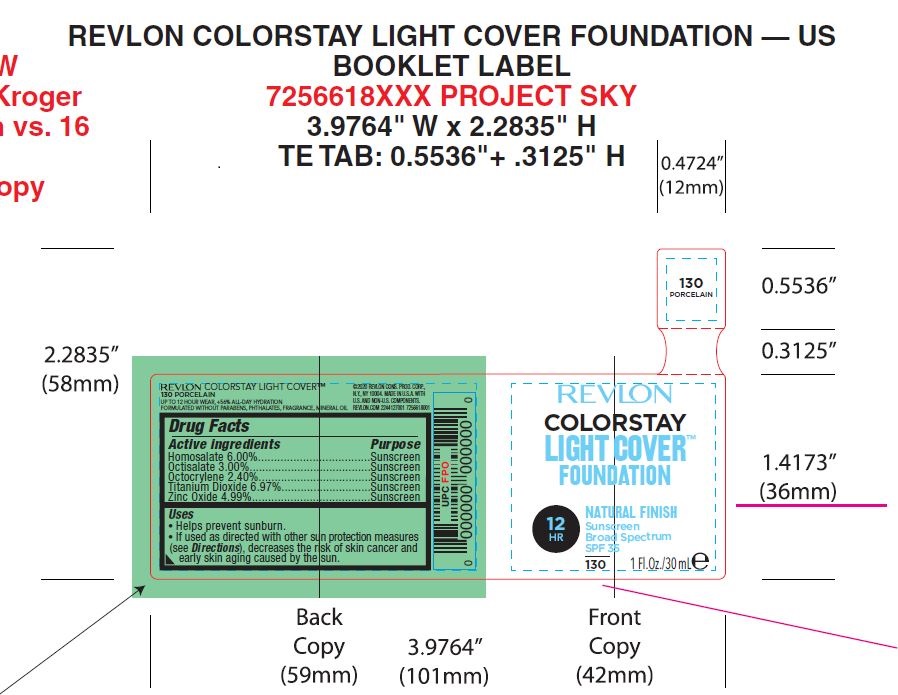
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
REVLON COLORSTAY LIGHT COVER FOUNDATION
homosalate, octisalate, octocrylene, titanium dioxide,zinc oxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-663 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 6.97 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 4.99 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 6 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 3 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) PROPYLENE CARBONATE (UNII: 8D08K3S51E) EDETATE SODIUM (UNII: MP1J8420LU) METHYL METHACRYLATE (UNII: 196OC77688) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (HARD PARTICLE) (UNII: H895X08VNQ) ALUMINUM OXIDE (UNII: LMI26O6933) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PROPANEDIOL (UNII: 5965N8W85T) NIACINAMIDE (UNII: 25X51I8RD4) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) CAFFEINE (UNII: 3G6A5W338E) DIMETHICONOL (14000 CST) (UNII: M2HW98ZA4V) METHICONE (20 CST) (UNII: 6777U11MKT) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PUNICA GRANATUM SEED OIL (UNII: 0UI45XV0T6) DIMETHICONE 100 (UNII: RO266O364U) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) ALCOHOL (UNII: 3K9958V90M) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) DIMETHICONE 1000 (UNII: MCU2324216) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) ISOCETETH-10 (UNII: 1K92T9919H) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MAGNESIUM SULFATE ANHYDROUS (UNII: ML30MJ2U7I) MICA (UNII: V8A1AW0880) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-663-01 1 mL in 1 TUBE; Type 0: Not a Combination Product 10/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2020 Labeler - Revlon (788820165) Establishment Name Address ID/FEI Business Operations Revlon INC. 809725570 manufacture(10967-663)