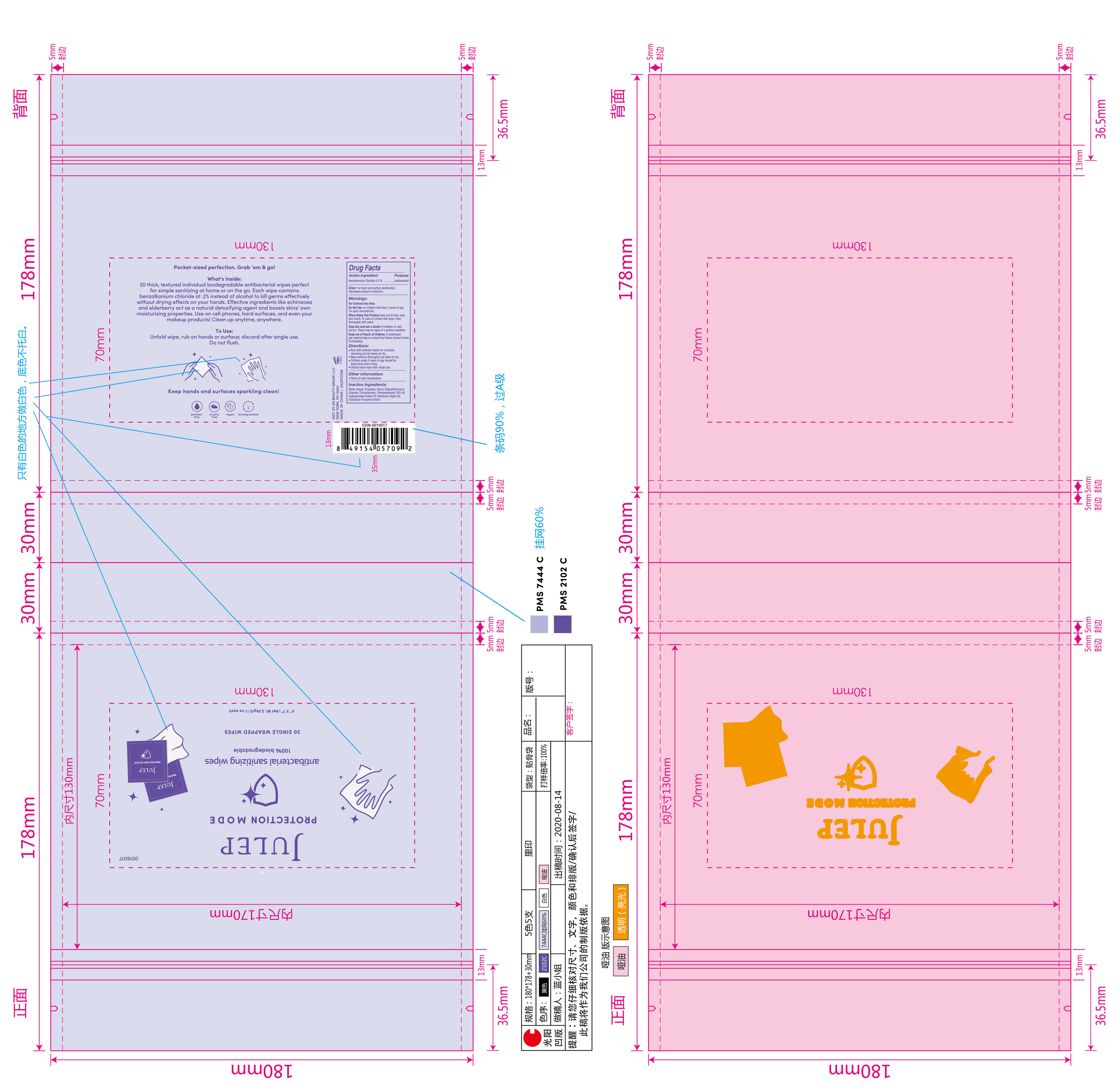
Label: JULEP ANTIBACTERIAL SANITIZING WIPE- benzalkonium chloride cloth
- NDC Code(s): 76635-121-01
- Packager: Hangzhou Glamcos Biotech CO.,LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
-
Warnings
For External Use Only.
Do Not Use on children less than 2 years of age.
On open wounds/cuts.
When Using This Product keep out of eyes,ears and mouth.In case of contact with eyes,rinse thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs.These may be signs of a serious condition. - On Children
- Directions
- Inactive ingredients
- Primay Face of Package
-
INGREDIENTS AND APPEARANCE
JULEP ANTIBACTERIAL SANITIZING WIPE
benzalkonium chloride clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76635-121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.2 g in 100 g Inactive Ingredients Ingredient Name Strength POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CHLORPHENESIN (UNII: I670DAL4SZ) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) DIDECYLDIMONIUM CHLORIDE (UNII: JXN40O9Y9B) ECHINACEA PURPUREA (UNII: QI7G114Y98) SAMBUCUS NIGRA SEED OIL (UNII: ZZV3NWA4A3) Product Characteristics Color Score Shape SQUARE (Hand Wipe Cloth) Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76635-121-01 3.24 g in 1 BAG; Type 0: Not a Combination Product 09/23/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 09/23/2020 Labeler - Hangzhou Glamcos Biotech CO.,LTD (554476017) Registrant - Hangzhou Glamcos Biotech CO.,LTD (554476017) Establishment Name Address ID/FEI Business Operations Hangzhou Glamcos Biotech CO.,LTD 554476017 manufacture(76635-121)