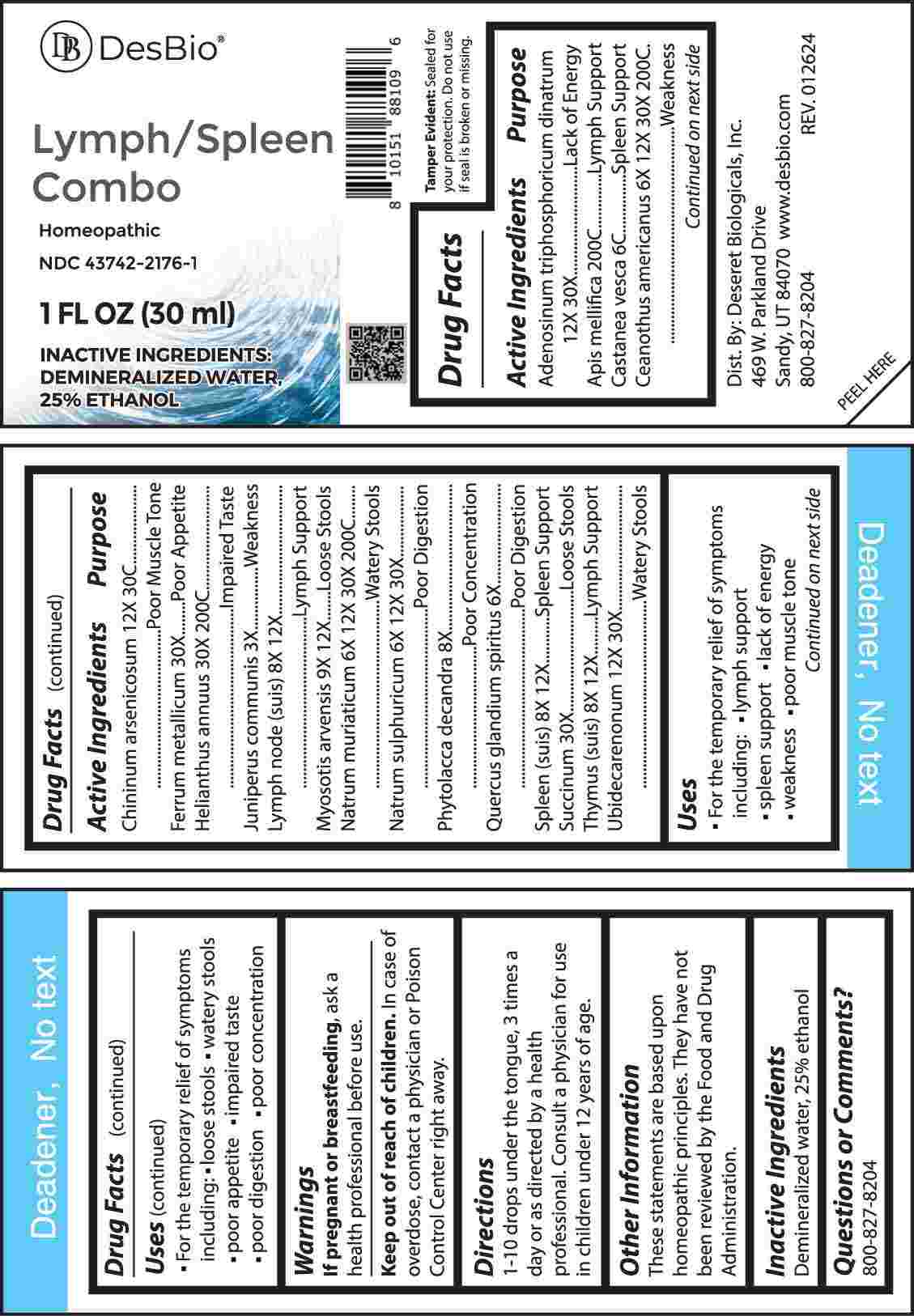
Label: LYMPH/SPLEEN COMBO (adenosinum triphosphoricum dinatrum, apis mellifica, castanea vesca, ceanothus americanus, chininum arsenicosum, ferrum metallicum, helianthus annuus, juniperus communis, lymph node (suis), myosotis arvensis, natrum muriaticum, natrum sulphuricum, phytolacca decandra, quercus glandium spiritus, spleen (suis), succinum, thymus- suis, ubidecarenonum liquid
- NDC Code(s): 43742-2176-1
- Packager: Deseret Biologicals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS:
Adenosinum Triphosphoricum Dinatrum 12X, 30X, Apis Mellifica 200C, Castanea Vesca 6C, Ceanothus Americanus 6X, 12X, 30X, 200C, Chininum Arsenicosum 12X, 30C, Ferrum Metallicum 30X, Helianthus Annuus 30X, 200C, Juniperus Communis 3X, Lymph Node (Suis) 8X, 12X, Myosotis Arvensis 9X, 12X, Natrum Muriaticum 6X, 12X, 30X, 200C, Natrum Sulphuricum 6X, 12X, 30X, Phytolacca Decandra 8X, Quercus Glandium Spiritus 6X, Spleen (Suis) 8X, 12X, Succinum 30X, Thymus (Suis) 8X, 12X, Ubidecarenonum 12X, 30X.
-
PURPOSE:
Adenosinum Triphosphoricum Dinatrum – Lack of Energy, Apis Mellifica – Lymph Support, Castanea Vesca – Spleen Support, Ceanothus Americanus - Weakness, Chininum Arsenicosum – Poor Muscle Tone, Ferrum Metallicum – Poor Appetite, Helianthus Annuus – Impaired Taste, Juniperus Communis - Weakness, Lymph Node (Suis) – Lymph Support, Myosotis Arvensis – Loose Stools, Natrum Muriaticum – Watery Stools, Natrum Sulphuricum – Poor Digestion, Phytolacca Decandra – Poor Concentration, Quercus Glandium Spiritus – Poor Digestion, Spleen (Suis) – Spleen Support, Succinum – Loose Stools, Thymus (Suis) – Lymph Support, Ubidecarenonum – Watery Stools
-
USES:
• For the temporary relief of symptoms including:
• lymph support • spleen support • lack of energy
• weakness • poor muscle tone • loose stools
• watery stools • poor appetite • impaired taste
• poor digestion • poor concentration
These statements are based upon homeopathic principles. They have not been reviewed by the Food and Drug Administration.
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
LYMPH/SPLEEN COMBO
adenosinum triphosphoricum dinatrum, apis mellifica, castanea vesca, ceanothus americanus, chininum arsenicosum, ferrum metallicum, helianthus annuus, juniperus communis, lymph node (suis), myosotis arvensis, natrum muriaticum, natrum sulphuricum, phytolacca decandra, quercus glandium spiritus, spleen (suis), succinum, thymus (suis), ubidecarenonum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43742-2176 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ADENOSINE TRIPHOSPHATE DISODIUM (UNII: 5L51B4DR1G) (ADENOSINE TRIPHOSPHATE - UNII:8L70Q75FXE) ADENOSINE TRIPHOSPHATE DISODIUM 12 [hp_X] in 1 mL APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 200 [hp_C] in 1 mL CASTANEA SATIVA LEAF (UNII: IV3S2HH53G) (CASTANEA SATIVA LEAF - UNII:IV3S2HH53G) CASTANEA SATIVA LEAF 6 [hp_C] in 1 mL CEANOTHUS AMERICANUS LEAF (UNII: 25B1Y14T8N) (CEANOTHUS AMERICANUS LEAF - UNII:25B1Y14T8N) CEANOTHUS AMERICANUS LEAF 6 [hp_X] in 1 mL QUININE ARSENITE (UNII: 42QO5P0NLM) (QUININE - UNII:A7V27PHC7A) QUININE ARSENITE 12 [hp_X] in 1 mL IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 30 [hp_X] in 1 mL HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) (HELIANTHUS ANNUUS FLOWERING TOP - UNII:BKJ0J3D1BP) HELIANTHUS ANNUUS FLOWERING TOP 30 [hp_X] in 1 mL JUNIPER BERRY (UNII: O84B5194RL) (JUNIPER BERRY - UNII:O84B5194RL) JUNIPER BERRY 3 [hp_X] in 1 mL SUS SCROFA LYMPH (UNII: 33A7VYU29L) (SUS SCROFA LYMPH - UNII:33A7VYU29L) SUS SCROFA LYMPH 8 [hp_X] in 1 mL MYOSOTIS ARVENSIS WHOLE (UNII: C73BK97H5J) (MYOSOTIS ARVENSIS - UNII:C73BK97H5J) MYOSOTIS ARVENSIS WHOLE 9 [hp_X] in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 6 [hp_X] in 1 mL SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 6 [hp_X] in 1 mL PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 8 [hp_X] in 1 mL QUERCUS ROBUR NUT (UNII: Q7MU1F4GLY) (QUERCUS ROBUR NUT - UNII:Q7MU1F4GLY) QUERCUS ROBUR NUT 6 [hp_X] in 1 mL SUS SCROFA SPLEEN (UNII: 92AMN5J79Y) (SUS SCROFA SPLEEN - UNII:92AMN5J79Y) SUS SCROFA SPLEEN 8 [hp_X] in 1 mL AMBER (UNII: 70J9Z0J26P) (AMBER - UNII:70J9Z0J26P) AMBER 30 [hp_X] in 1 mL SUS SCROFA THYMUS (UNII: 7B69B0BD62) (SUS SCROFA THYMUS - UNII:7B69B0BD62) SUS SCROFA THYMUS 8 [hp_X] in 1 mL UBIDECARENONE (UNII: EJ27X76M46) (UBIDECARENONE - UNII:EJ27X76M46) UBIDECARENONE 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43742-2176-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 07/31/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/31/2024 Labeler - Deseret Biologicals, Inc. (940741853) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(43742-2176) , api manufacture(43742-2176) , label(43742-2176) , pack(43742-2176)