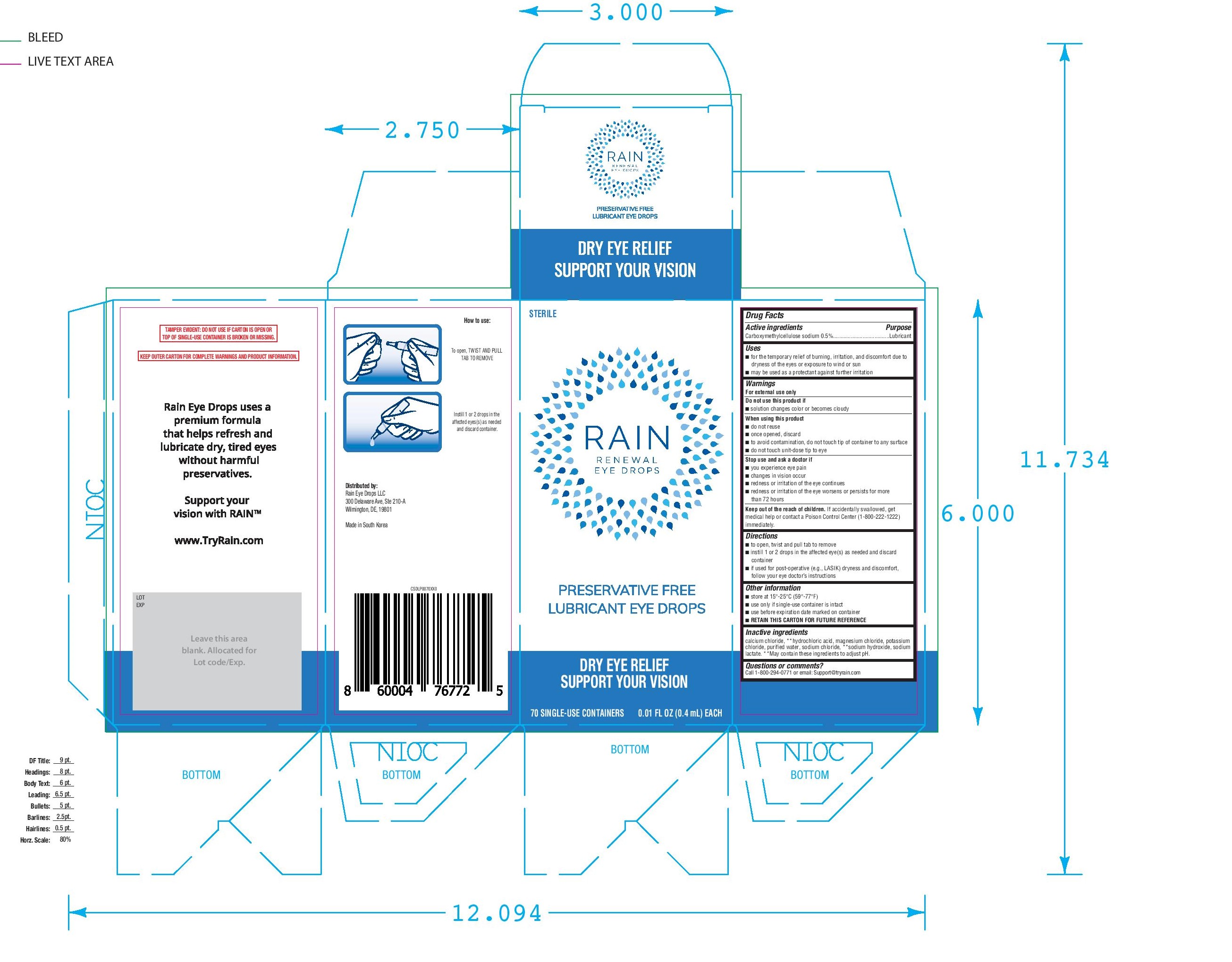
Label: RAIN RENEWAL EYE DROPS- carboxymethylcellulose sodium solution/ drops
- NDC Code(s): 79662-002-70
- Packager: Rain Eye Drops, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- 70 ct
-
INGREDIENTS AND APPEARANCE
RAIN RENEWAL EYE DROPS
carboxymethylcellulose sodium solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79662-002 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) (CARBOXYMETHYLCELLULOSE - UNII:05JZI7B19X) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM 0.5 g in 100 mL Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) WATER (UNII: 059QF0KO0R) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SODIUM LACTATE (UNII: TU7HW0W0QT) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79662-002-70 70 in 1 BOX 09/18/2020 11/30/2023 1 0.4 mL in 1 VIAL, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 09/18/2020 Labeler - Rain Eye Drops, LLC (117579363)