Label: KLEAN-HANDS liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 73943-948-01, 73943-948-02, 73943-948-03, 73943-948-04, view more73943-948-05 - Packager: Rite-Kem Incorporated
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 14, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- COMPONENTS
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
-
Label
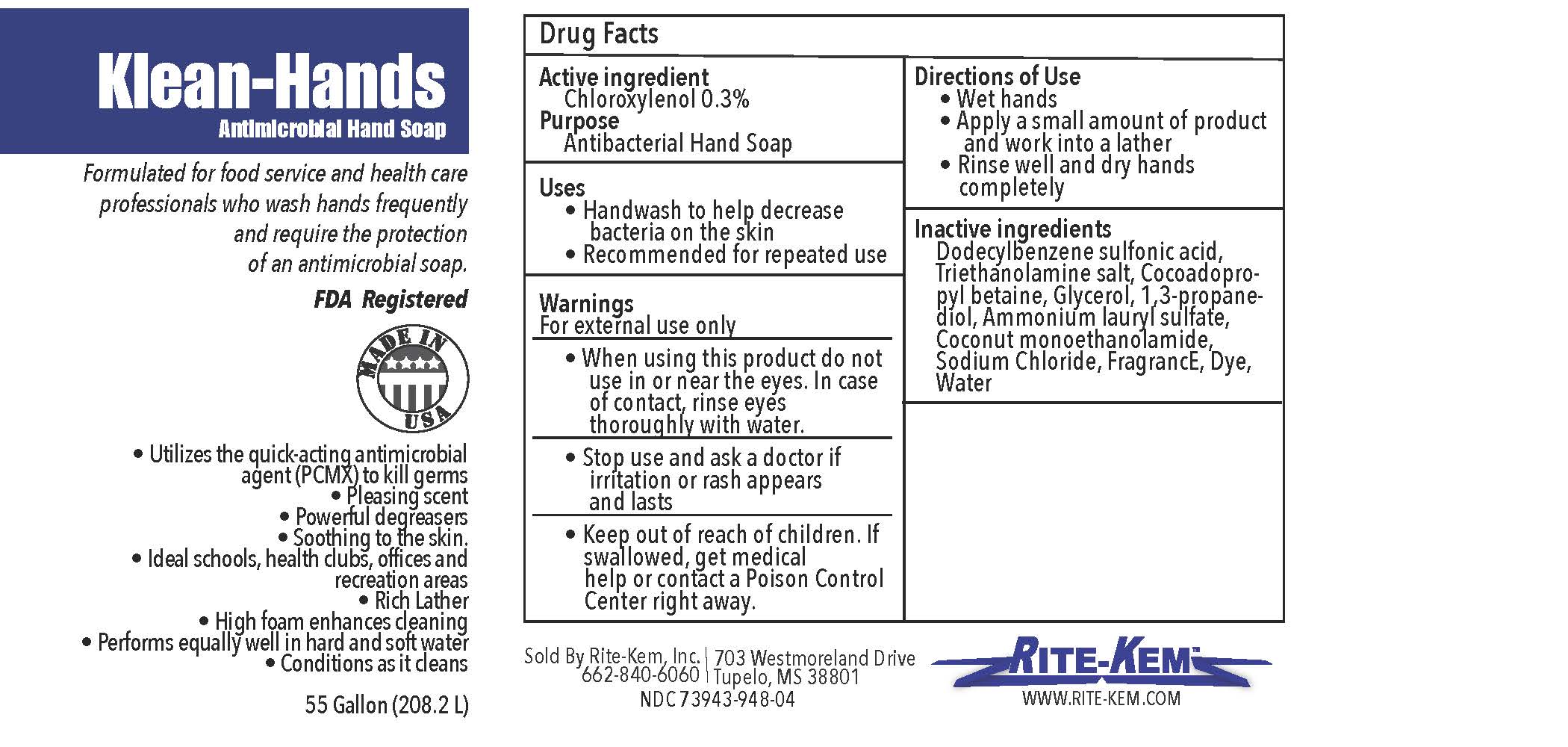
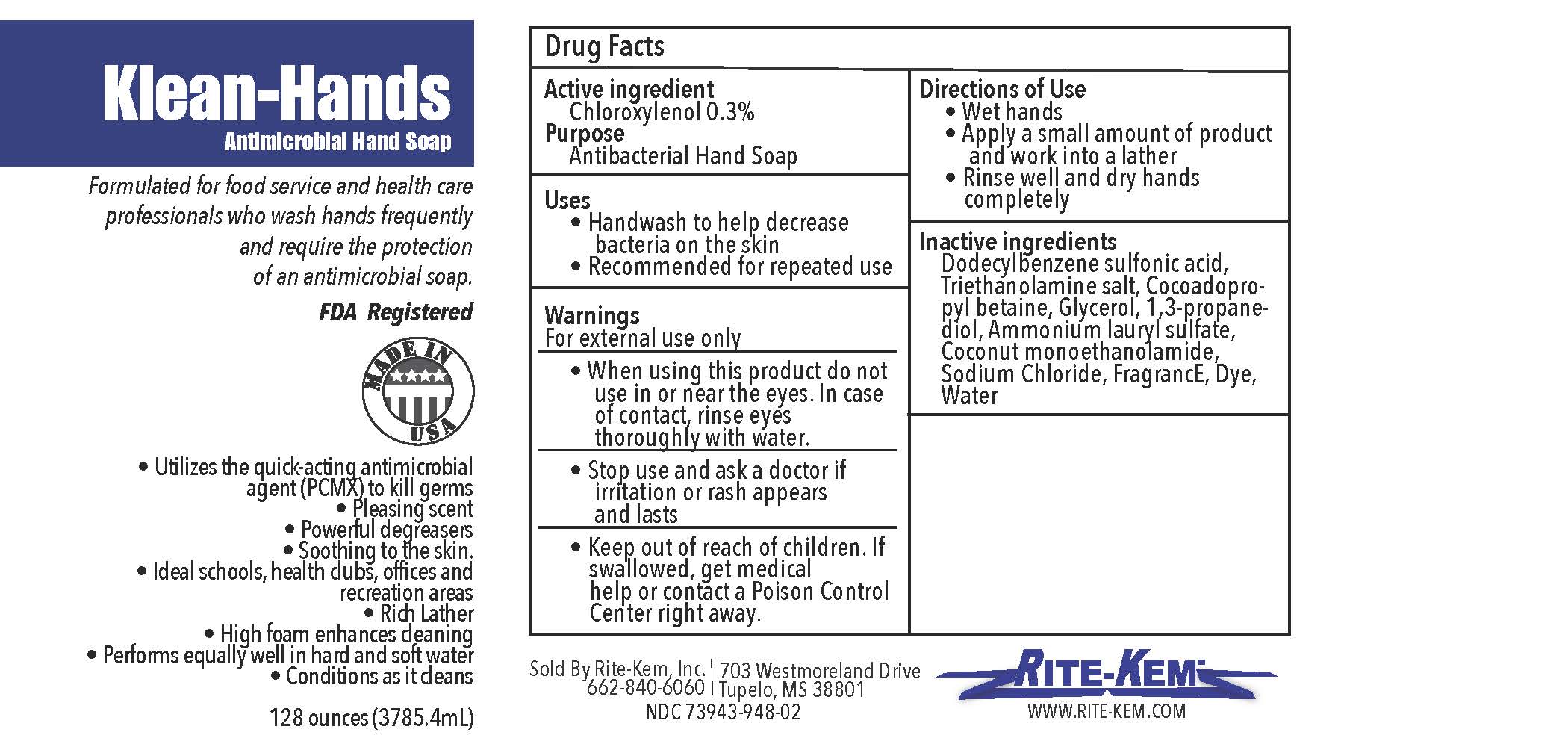
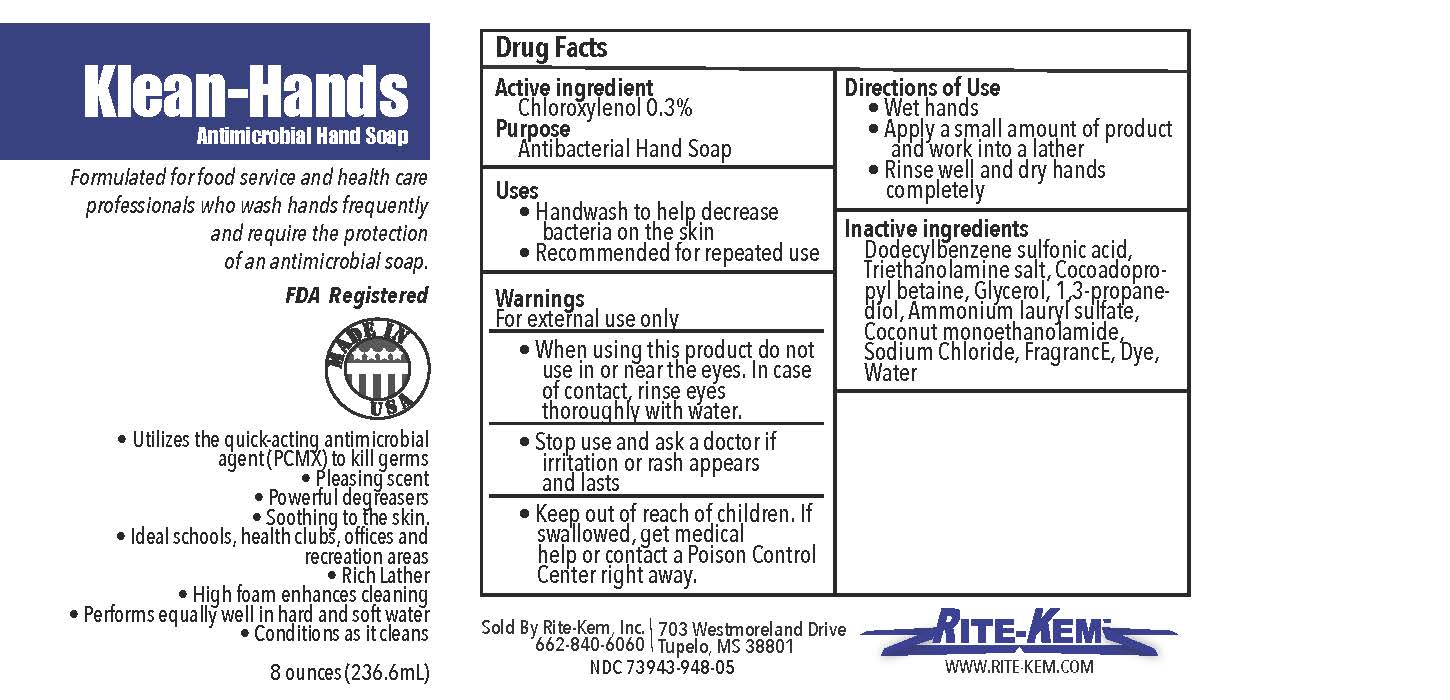
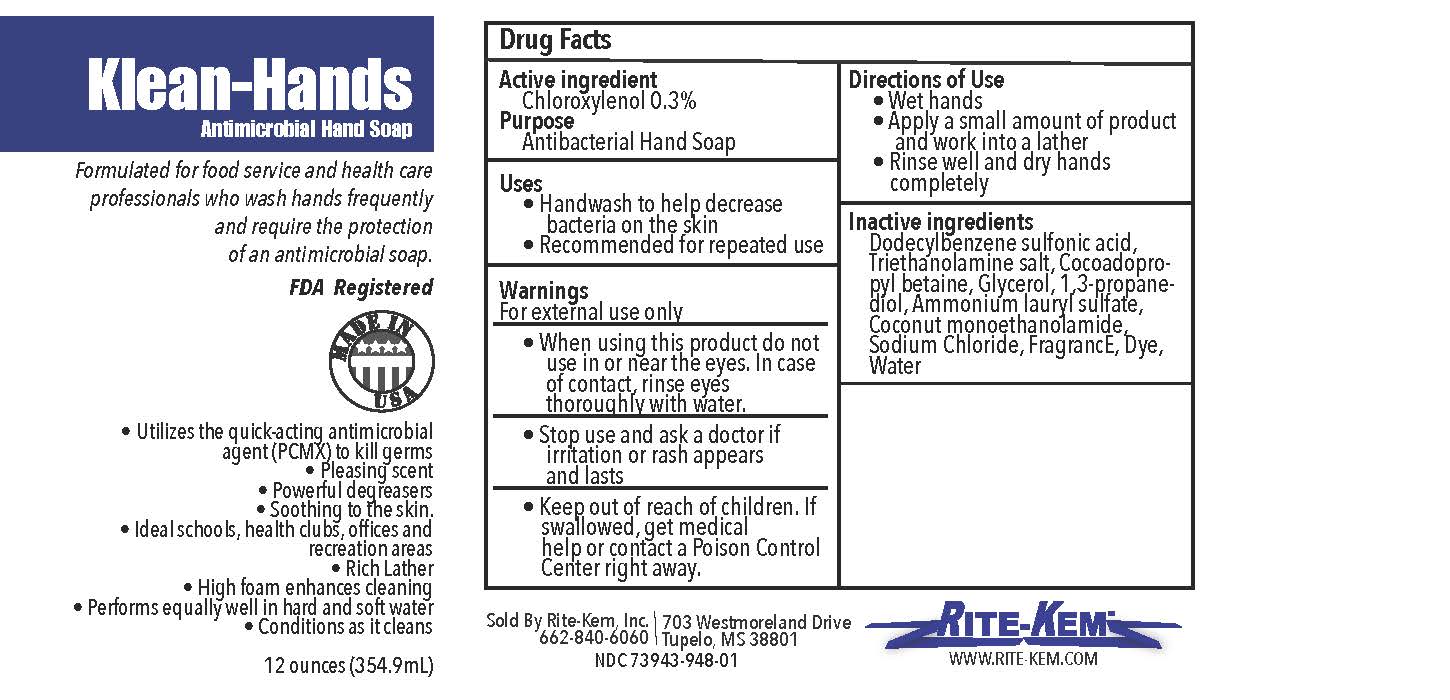
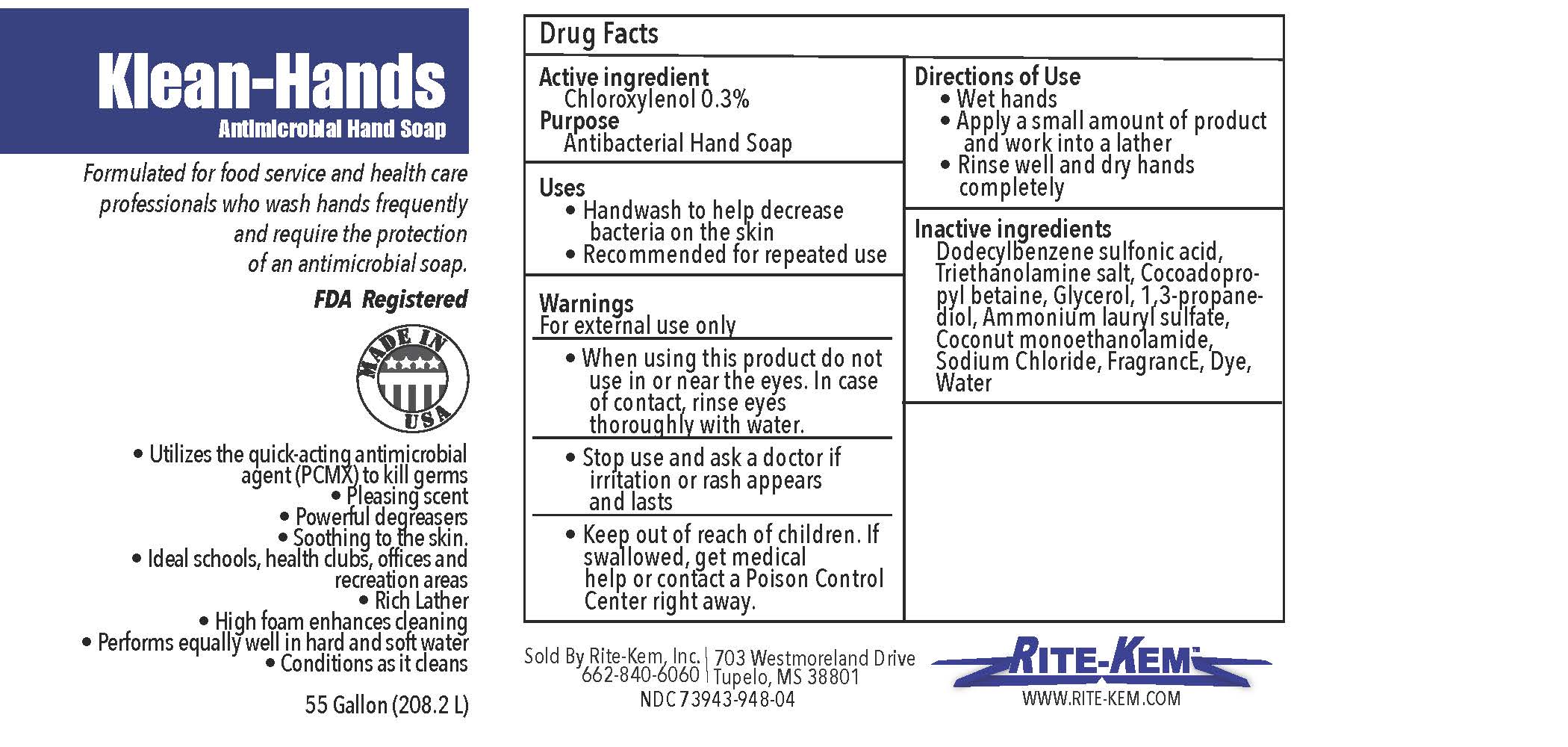
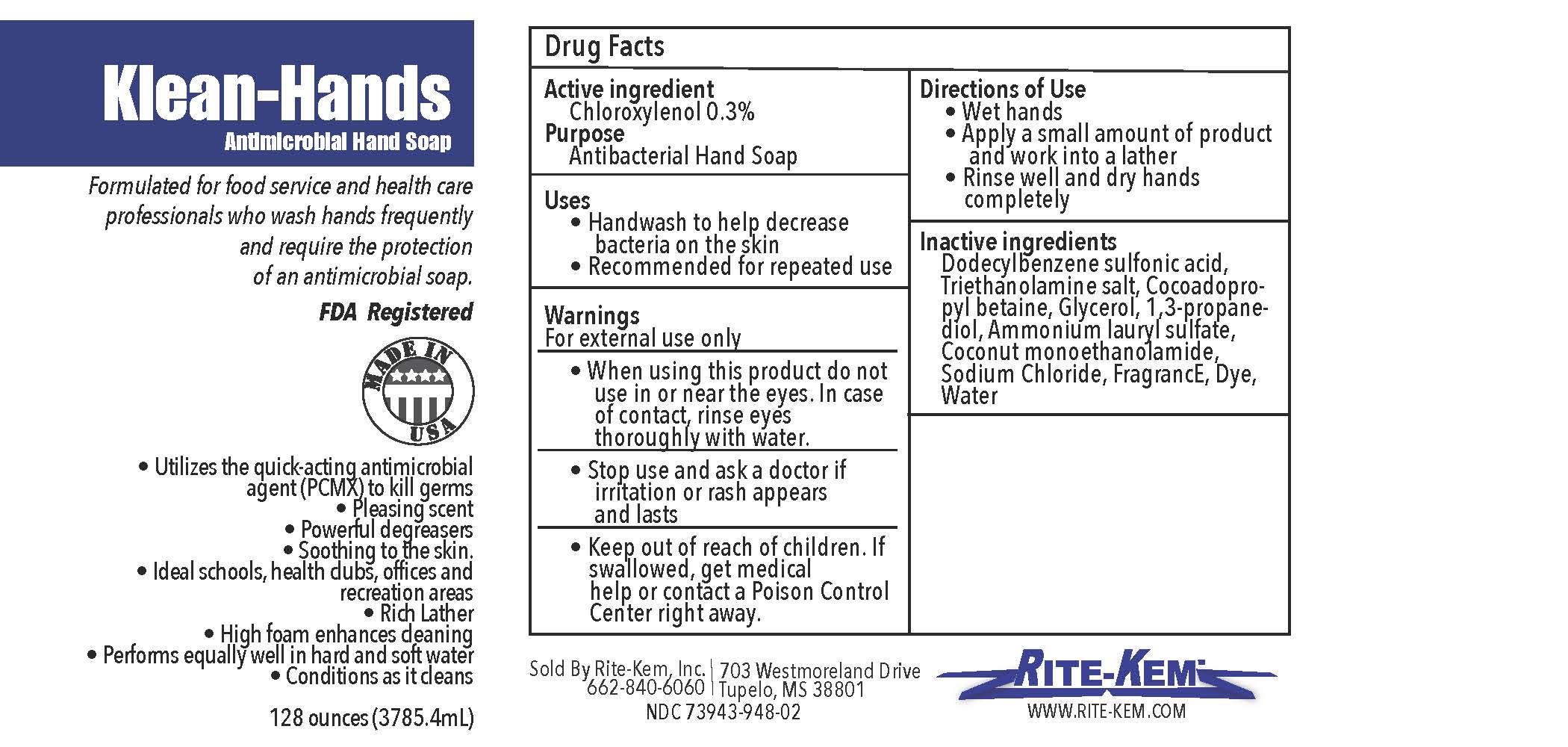
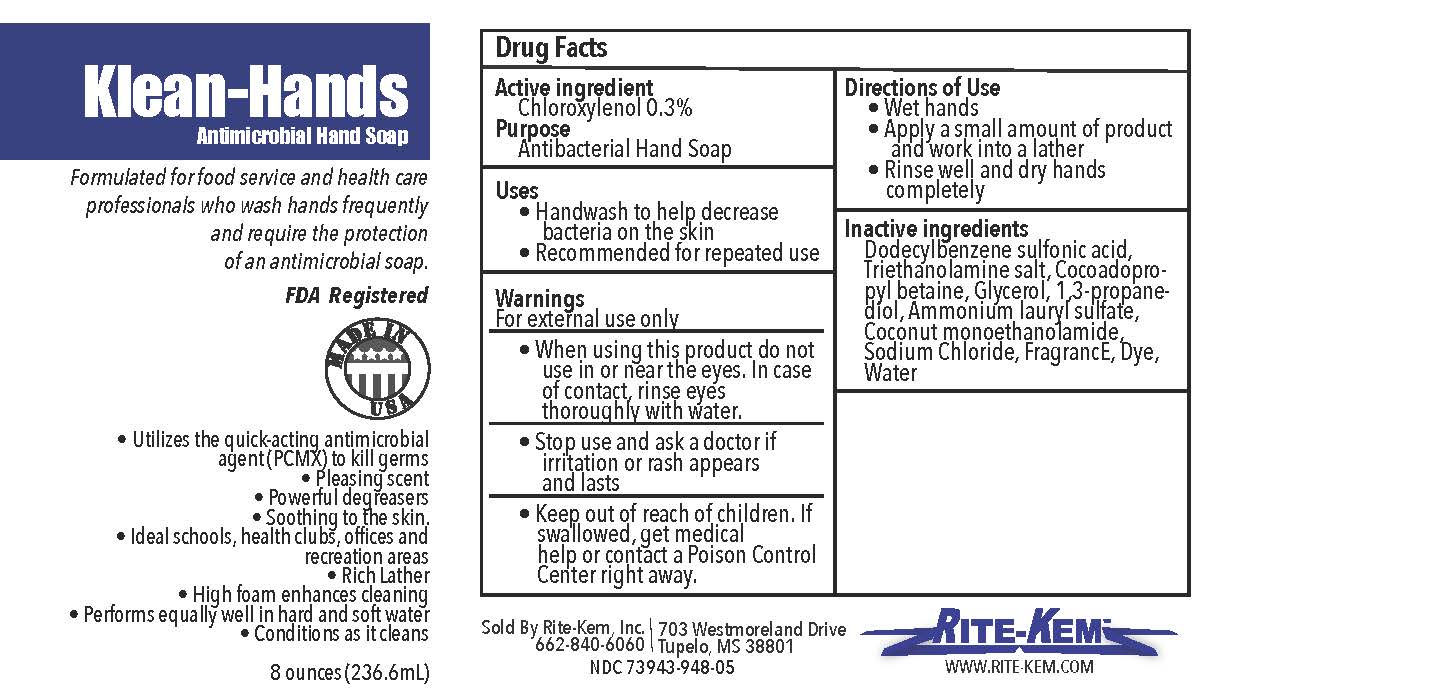
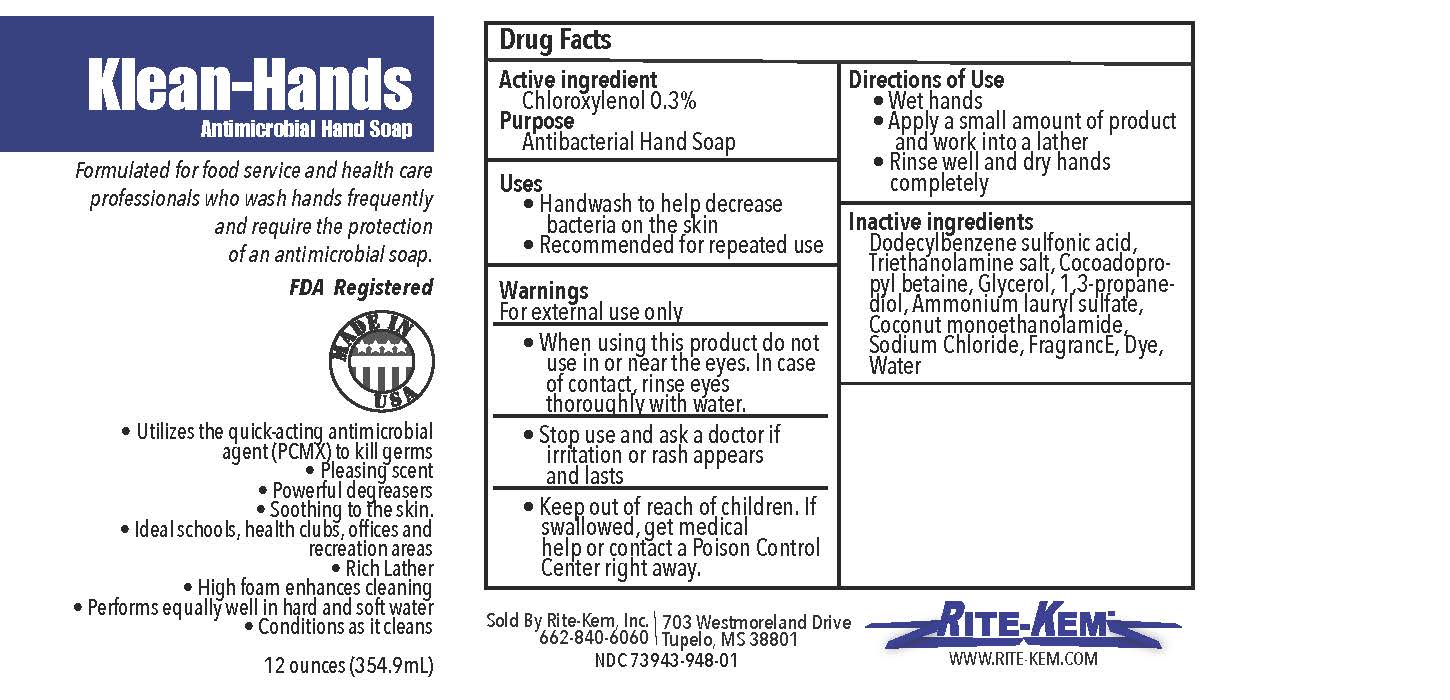
Klean-Hands Antimicrobial Hand soap
Formulated for food service and health care professionals who wash hands frequently and require the protection of an antimicrobial soap.
FDA registered
Made in USA
* Utilize the quickacting antimicrobial agent (PCMX) to kill germs
* Pleasing agent
* Powerful degreasers
* Soothing to the skin
* Ideal schools, health clubs, offices and recreation areas
* Rich Lather
* High foam enhances cleaning
* Performs equally well in hand and soft water
* Conditions as it cleans
Sold by: Rite-Kem Inc., 703 Westmoreland Drive, Tupelco, MS 38801
Ph.: 662-840-6060
NDC: 73943-948-xx

-
INGREDIENTS AND APPEARANCE
KLEAN-HANDS
klean-hands liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73943-948 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 0.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROLAMINE (UNII: 9O3K93S3TK) GLYCERIN (UNII: PDC6A3C0OX) SODIUM CHLORIDE (UNII: 451W47IQ8X) COCO MONOETHANOLAMIDE (UNII: C80684146D) LAVENDER OIL (UNII: ZBP1YXW0H8) PROPANEDIOL (UNII: 5965N8W85T) AMMONIUM LAURYL SULFATE (UNII: Q7AO2R1M0B) DODECYLBENZENESULFONIC ACID (UNII: 60NSK897G9) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73943-948-01 354 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/18/2020 2 NDC:73943-948-02 3785 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/18/2020 3 NDC:73943-948-03 18927 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/18/2020 4 NDC:73943-948-04 208198 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/18/2020 5 NDC:73943-948-05 236 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/08/2021 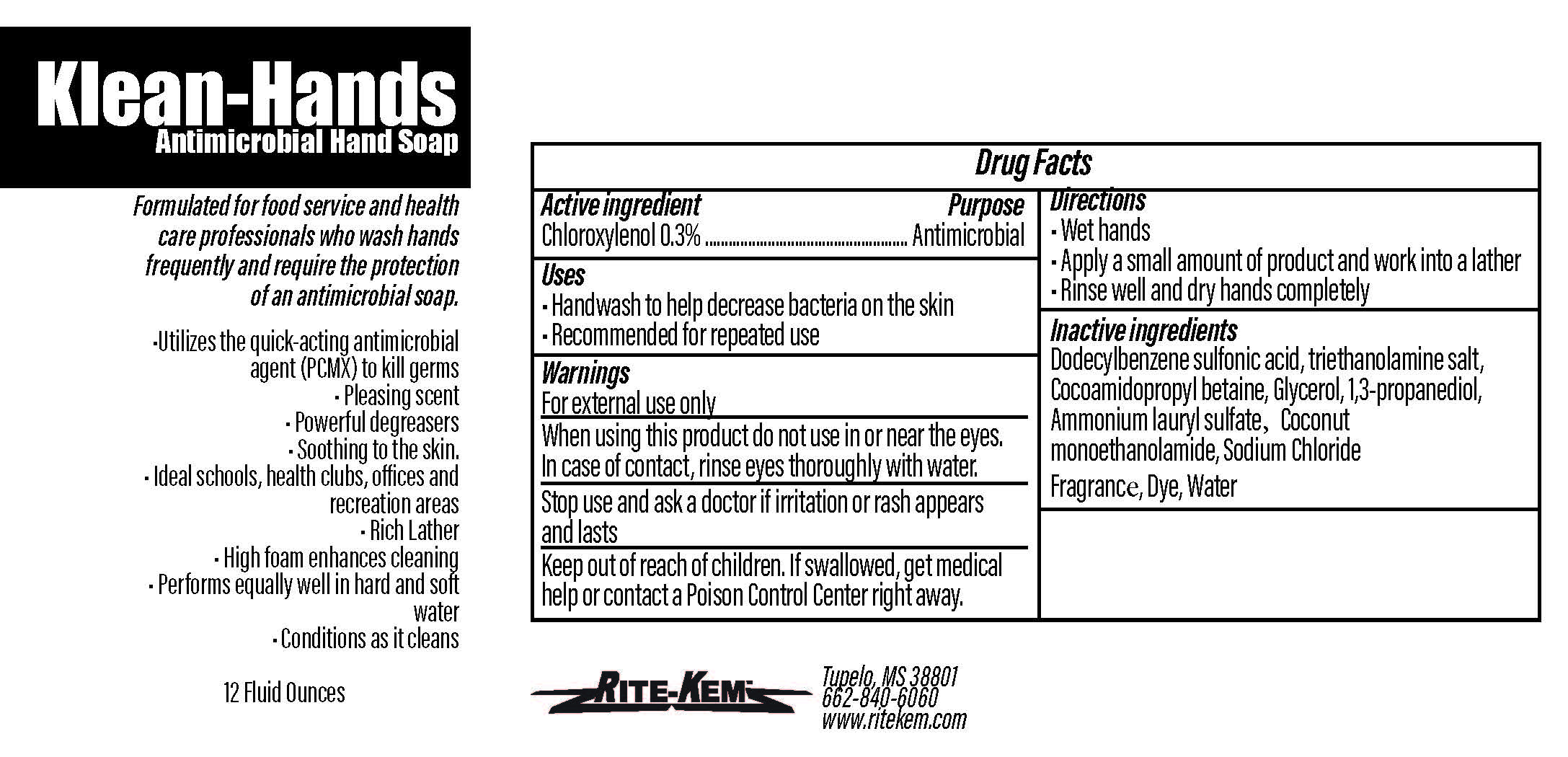
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/18/2020 Labeler - Rite-Kem Incorporated (786892927) Establishment Name Address ID/FEI Business Operations Rite-Kem Incorporated 786892927 manufacture(73943-948)