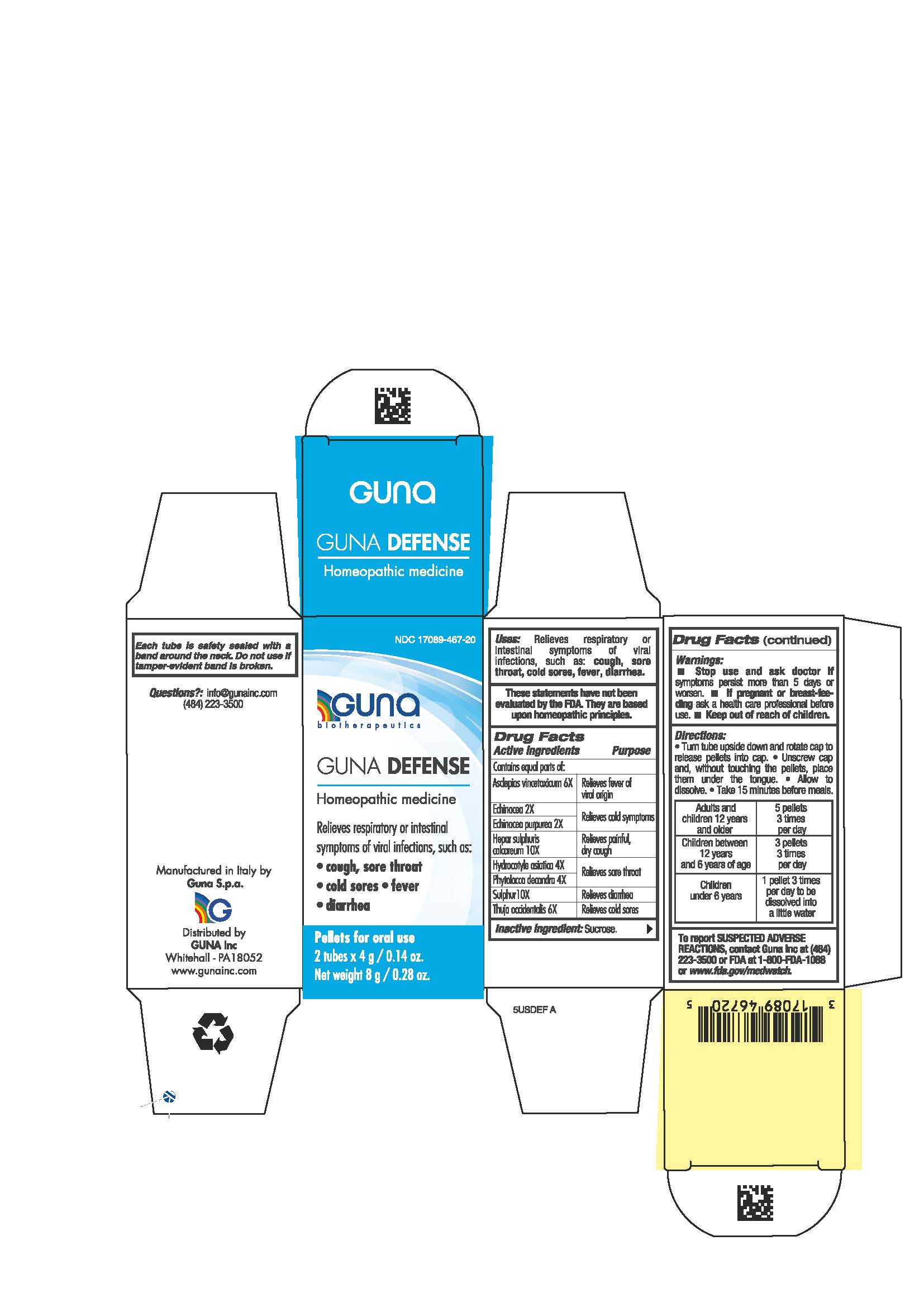
Label: GUNA DEFENSE- calcium sulfide - centella asiatica - echinacea angustifolia whole - echinacea purpurea - thuja occidentalis leafy twig - vincetoxicum hirundinaria root - phytolacca americana root - sulfur - pellet
- NDC Code(s): 17089-467-20, 17089-467-21
- Packager: Guna spa
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 15, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- QUESTIONS
- DIRECTIONS
- WARNINGS
- PREGNANCY
- WARNINGS
- USES
-
ACTIVE INGREDIENTS/PURPOSE
Asclepias vincetoxicum 6X Relieves fever of viral origin
Echinacea 2X Relieves cold symptoms
Echinacea purpurea 2X Relieves cold symptoms
Hepar sulphuris calc. 10X Relieves painful, dry cough
Hydrocotyle asiatica 4X Relieves sore throat
Phytolacca decandra 4X Relieves sore throat
Sulphur 10X Relieves diarrhea
Thuja occidentalis 6X Relieves cold sores
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA DEFENSE
calcium sulfide - centella asiatica - echinacea angustifolia whole - echinacea purpurea - thuja occidentalis leafy twig - vincetoxicum hirundinaria root - phytolacca americana root - sulfur - pelletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-467 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CENTELLA ASIATICA (UNII: 7M867G6T1U) (CENTELLA ASIATICA - UNII:7M867G6T1U) CENTELLA ASIATICA 4 [hp_X] in 4 g ECHINACEA ANGUSTIFOLIA WHOLE (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA WHOLE - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA WHOLE 2 [hp_X] in 4 g ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA - UNII:QI7G114Y98) ECHINACEA PURPUREA 2 [hp_X] in 4 g CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 10 [hp_X] in 4 g THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 6 [hp_X] in 4 g VINCETOXICUM HIRUNDINARIA ROOT (UNII: 9R858U917W) (VINCETOXICUM HIRUNDINARIA ROOT - UNII:9R858U917W) VINCETOXICUM HIRUNDINARIA ROOT 6 [hp_X] in 4 g PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 4 [hp_X] in 4 g SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 10 [hp_X] in 4 g Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) 4 g in 4 g Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-467-20 2 in 1 BOX 09/24/2020 1 4 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:17089-467-21 1 in 1 BOX 12/15/2021 2 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/24/2020 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-467)