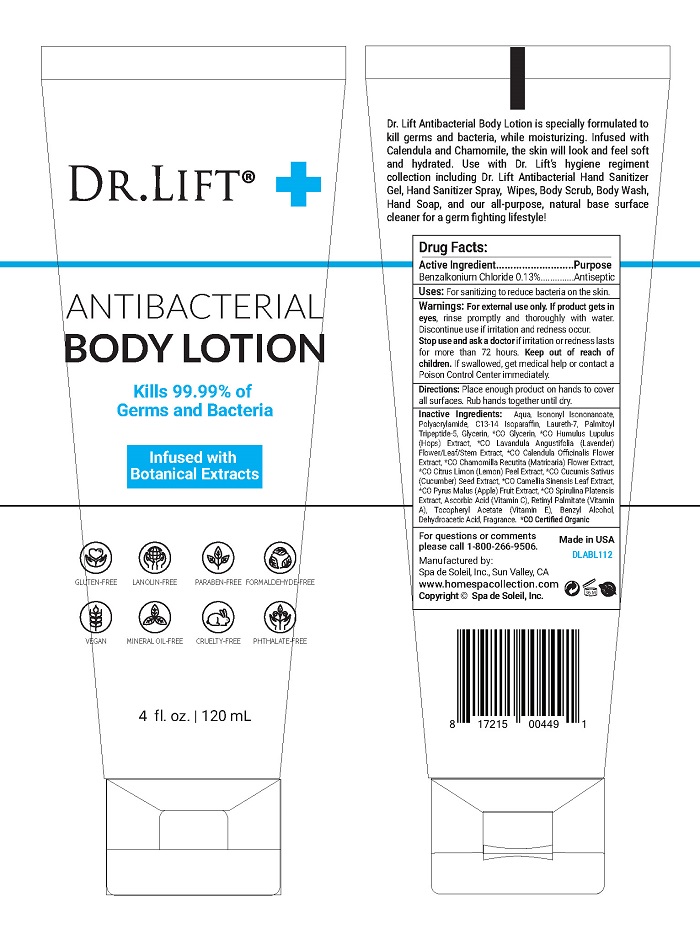
Label: DR LIFT ANTIBACTERIAL- benzalkonium chloride lotion
- NDC Code(s): 68062-2243-1
- Packager: Spa de Soleil
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 17, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
-
Warnings
Warnings
For external use only. If product gets in eyes, rinse promptly and thoroughly with water. Discontinue use if irritation and redness occur.
Stop use and ask a doctor if irritation or redness lasts for more than 72 hours. Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
- KEEP OUT OF REACH OF CHILDREN
- Directions
-
Inactive Ingredients
Inactive Ingredients:
Aqua, Isononyl Isononanoate, Polyacrylamide, C13-14 Isoparaffin, Laureth-7, Palmitoyl Tripeptide-5, Glycerin, *CO Glycerin, *CO Humulus Lupulus (Hops) Extract, *CO Lavandula Angustifolia (Lavender) Flower/Leaf/Stem Extract, *CO Calendula Officinalis Flower Extract, *CO Chamomilla Recutita (Matricaria) Flower Extract, *CO Citrus Limon (Lemon) Peel Extract, *CO Cucumis Sativus (Cucumber) Seed Extract, *CO Camellia Sinensis Leaf Extract, *CO Pyrus Malus (Apple) Fruit Extract, *CO Spirulina Platensis Extract, Ascorbic Acid (Vitamin C), Retinyl Palmitate (Vitamin A), Tocopheryl Acetate (Vitamin E), Benzyl Alcohol, Dehydroacetic Acid, Fragrance. *CO Certified Organic
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DR LIFT ANTIBACTERIAL
benzalkonium chloride lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68062-2243 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.156 mg in 120 mg Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68062-2243-1 120 mg in 1 BOTTLE; Type 0: Not a Combination Product 09/17/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/17/2020 Labeler - Spa de Soleil (874682867) Establishment Name Address ID/FEI Business Operations Spa de Soleil 874682867 manufacture(68062-2243)