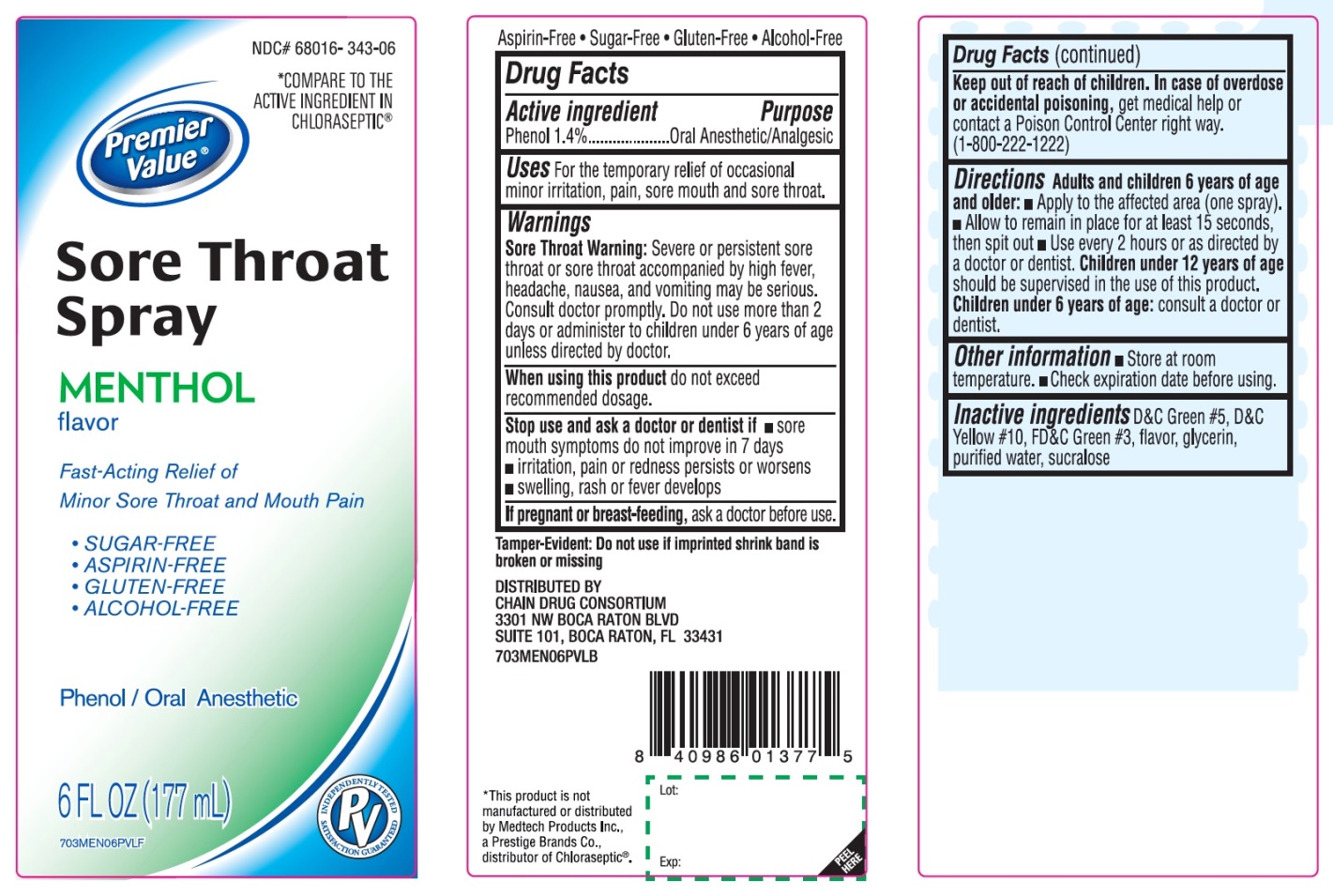
Label: SORE THROAT RELIEF MENTHOL- phenol spray
- NDC Code(s): 68016-343-06
- Packager: Chain Drug Consortium
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
Sore throat warning: Severe or persistent sore throat or sore throat that occurs with high fever, headache, nausea, and vomiting may be serious. Ask a doctor right away. Do not use more than 2 days or give to children under 3 years of age.
-
Directions
- Adults and children 6 years of age and older:
- •
- Apply to the affected area (one spray)
- •
- Allow to remain in place for at least 15 seconds, then spit out
- •
- Use every 2 hours or as directed by a doctor
- Children under 12 years of age should be supervised in the use of this product
- Children under 6 years of age: consult a doctor or dentist
- Other information
- Inactive ingredients
-
Principal Display Panel
*Compare to the active ingredient in Chloraseptic®*
Sore Throat Relief
Menthol Flavor
Fast acting relief of Minor Sore Throat and Mouth Pain
Phenol/Oral Anesthetic
Sugar-Free
Aspirin-Free
Gluten-Free
Alcohol-Free
6 FL OZ (177 mL)
*This product is not manufactured or distributed by Medtech Products Inc., a Prestige Brands Co., distributor of Chloraseptic®.
Tamper-Evident: Do not use if imprinted shrink band is broken or missing.
DISTRIBUTED BY:
Chain Drug Consortium LLC.
3301 N.W. Boca Raton Blvd.
Suite 101, Boca Raton FL 33431
- Package Label
-
INGREDIENTS AND APPEARANCE
SORE THROAT RELIEF MENTHOL
phenol sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68016-343 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENOL (UNII: 339NCG44TV) (PHENOL - UNII:339NCG44TV) PHENOL 1.4 g in 100 mL Inactive Ingredients Ingredient Name Strength D&C GREEN NO. 5 (UNII: 8J6RDU8L9X) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color GREEN Score Shape Size Flavor MENTHOL Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68016-343-06 177 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 12/31/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 12/31/2014 Labeler - Chain Drug Consortium (101668460)