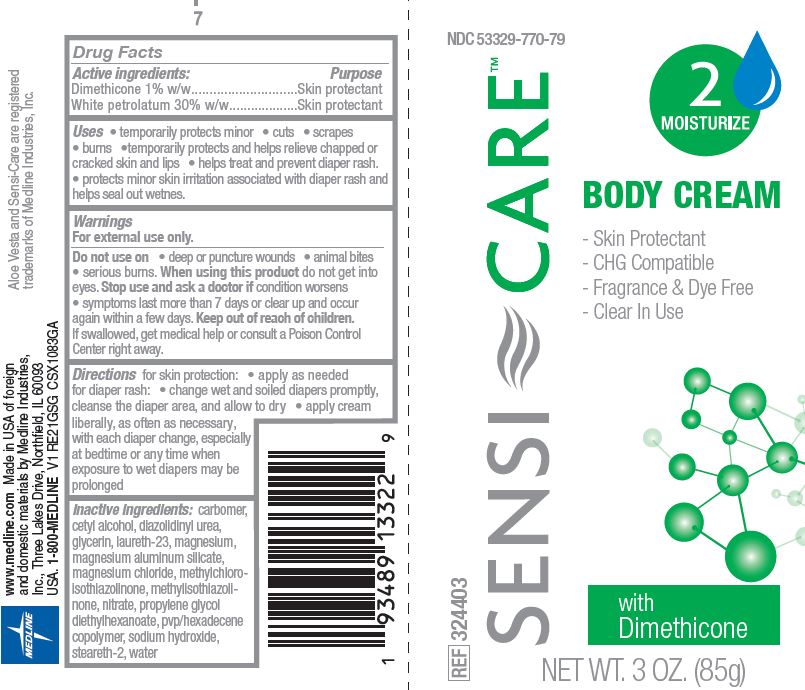
Label: SENSI-CARE- petrolatum, dimethicone cream
- NDC Code(s): 53329-770-79
- Packager: Medline Industries, LP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- Manufacturing Information
- Package Label
-
INGREDIENTS AND APPEARANCE
SENSI-CARE
petrolatum, dimethicone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53329-770 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 1 g in 100 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 30 g in 100 g Inactive Ingredients Ingredient Name Strength HEXADECENE (MIXED ISOMERS) (UNII: 38H8547VP0) STEARETH-2 (UNII: V56DFE46J5) WATER (UNII: 059QF0KO0R) MAGNESIUM (UNII: I38ZP9992A) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) NITRATE ION (UNII: T93E9Y2844) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) CETYL ALCOHOL (UNII: 936JST6JCN) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) GLYCERIN (UNII: PDC6A3C0OX) LAURETH-23 (UNII: N72LMW566G) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) PROPYLENE GLYCOL DIETHYLHEXANOATE (UNII: 8D8I9Z0F1Z) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53329-770-79 85 g in 1 TUBE; Type 0: Not a Combination Product 09/30/2020 04/30/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 09/30/2020 04/30/2025 Labeler - Medline Industries, LP (025460908) Registrant - Medline Industries, LP (025460908)