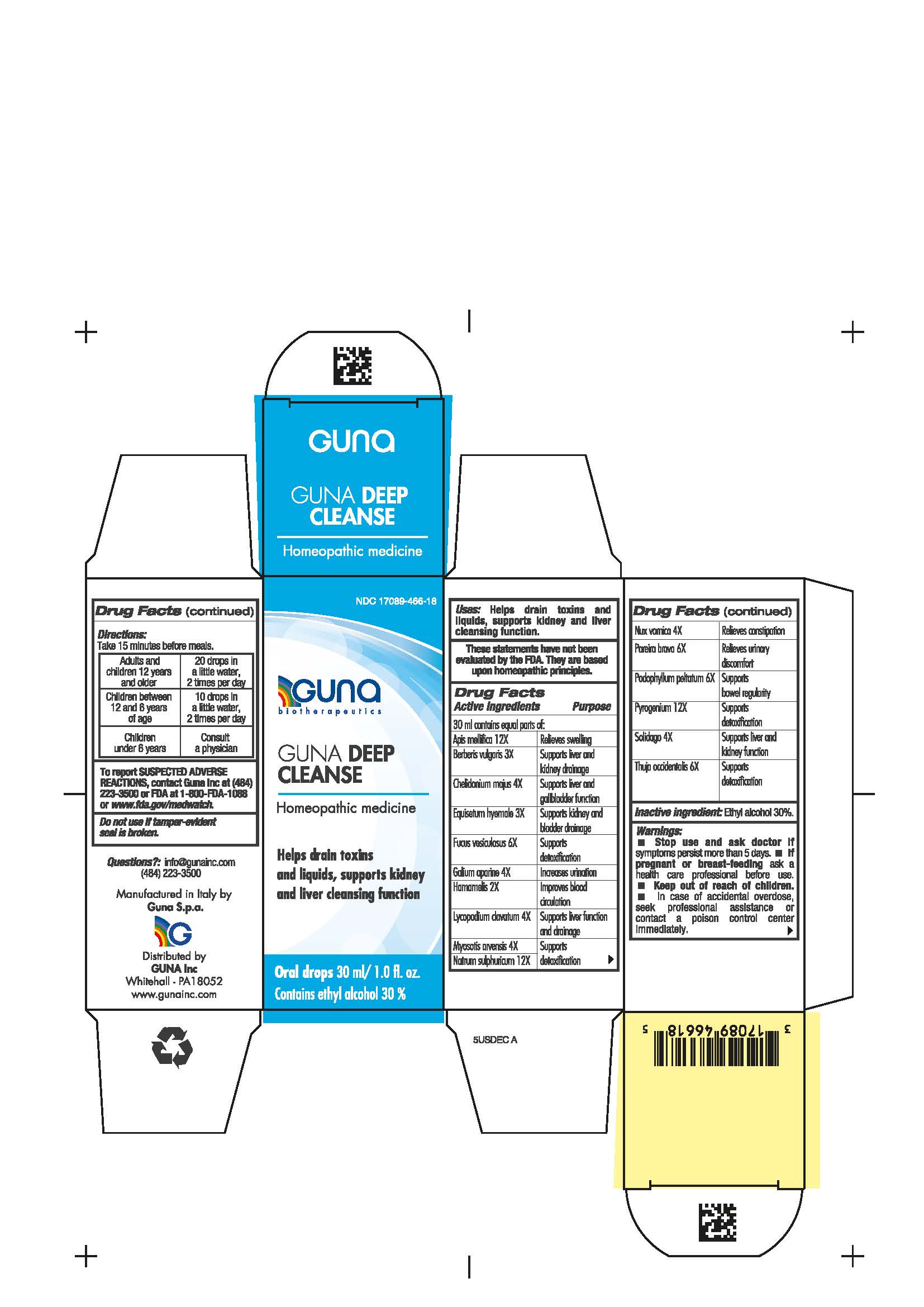
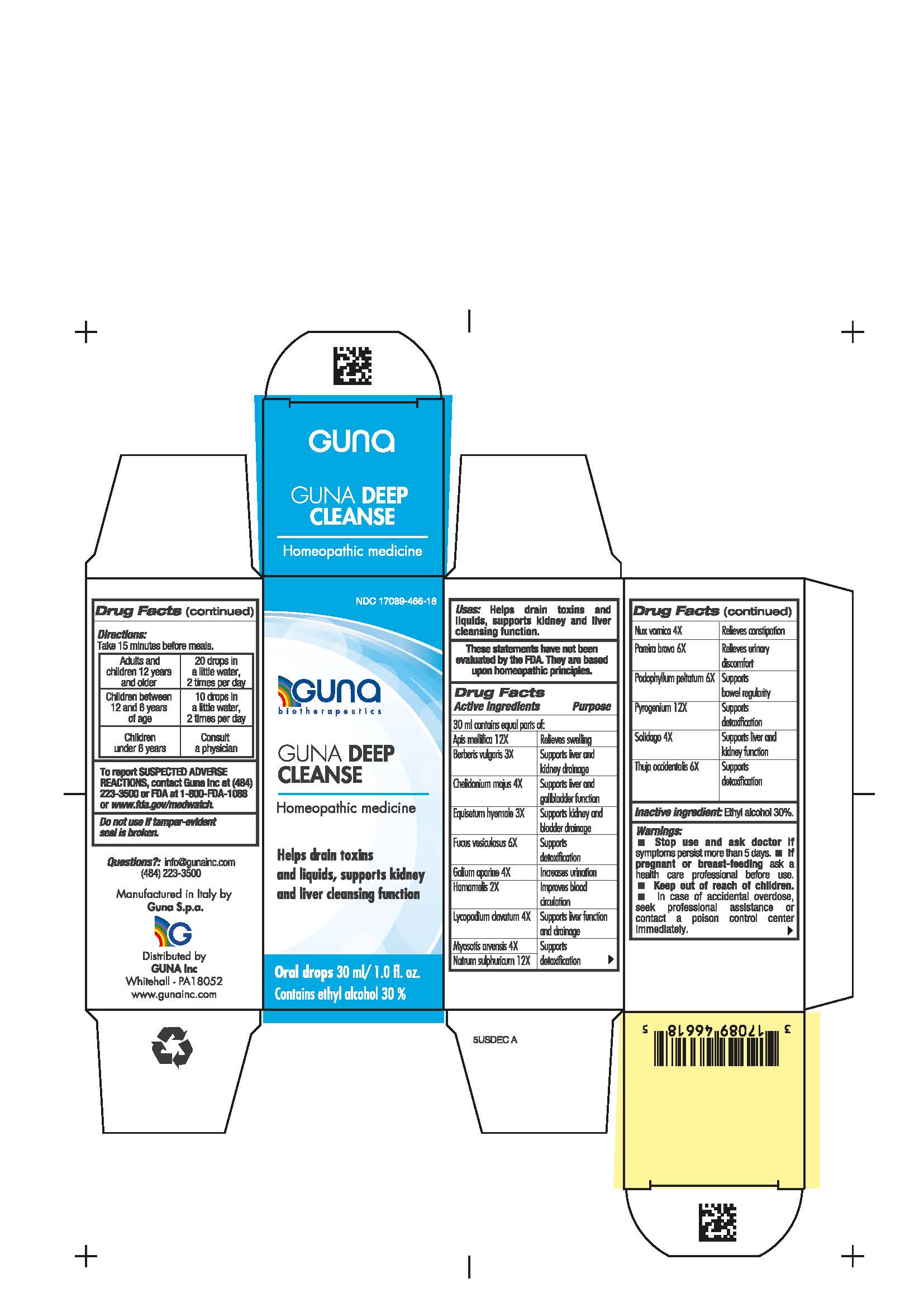
Label: GUNA DEEP CLEANSE- apis mellifera - berberis vulgaris root bark - chelidonium majus - chondrodendron tomentosum root - equisetum hyemale whole - fucus vesiculosus - galium aparine whole - hamamelis virginiana root bark - lycopodium clavatum spore - myosotis arvensis - podophyllum peltatum root - rancid beef - sodium sulfate - solidago virgaurea flowering top - strychnos nux vomica seed - thuja occidentalis leafy twig - solution/ drops
- NDC Code(s): 17089-466-18
- Packager: Guna spa
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated September 24, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WHEN USING
- Purpose
-
Active Ingredient(s)
Apis mellifica 12X Relieves swelling
Berberis vulgaris 3X Supports liver and kidney drainage
Chelidonoium majus 4X Supports liver and gallbladder function
Equisetum hyemale 3X Supports kidney and bladder drainage
Fucus vesiculosus 6X Supports detoxification
Galium aparine 4X Increases urination
Hamamelis 2X Improves blood circulation
Lycopodium clavatum 4X Supports liver function and drainage
Myosotis arvensis 4X Supports detoxification
Natrum sulphuricum 12X Supports detoxification
Nux vomica 4X Relieves constipation
Pareira brava 6X Relieves urinary discomfort
Podophyllum peltatum 6X Supports bowel regularity
Pyrogenium 12X Supports detoxification
Solidago 4X Supports liver and kidney function
Thuja occidentalis 6X Supports detoxification
- INDICATIONS & USAGE
-
Warnings
Warnings:
- Stop use and ask doctor if symptoms persist more than 5 days.
- If pregnant or breast-feeding ask a health professional before use.
- Keep out of reach of children.
- In case of accidental overdose, seek prfessional assistance or contact a Poison Control Center immediately
- Contains ethyl alcohol 30%
- Do not use
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- ADVERSE REACTIONS
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
GUNA DEEP CLEANSE
apis mellifera - berberis vulgaris root bark - chelidonium majus - chondrodendron tomentosum root - equisetum hyemale whole - fucus vesiculosus - galium aparine whole - hamamelis virginiana root bark - lycopodium clavatum spore - myosotis arvensis - podophyllum peltatum root - rancid beef - sodium sulfate - solidago virgaurea flowering top - strychnos nux vomica seed - thuja occidentalis leafy twig - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-466 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHELIDONIUM MAJUS ROOT (UNII: FLT36UCF0N) (CHELIDONIUM MAJUS ROOT - UNII:FLT36UCF0N) CHELIDONIUM MAJUS ROOT 4 [hp_X] in 30 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 4 [hp_X] in 30 mL SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM SULFATE 12 [hp_X] in 30 mL SOLIDAGO VIRGAUREA FLOWERING TOP (UNII: 5405K23S50) (SOLIDAGO VIRGAUREA FLOWERING TOP - UNII:5405K23S50) SOLIDAGO VIRGAUREA FLOWERING TOP 4 [hp_X] in 30 mL RANCID BEEF (UNII: 29SUH5R3HU) (RANCID BEEF - UNII:29SUH5R3HU) RANCID BEEF 12 [hp_X] in 30 mL FUCUS VESICULOSUS (UNII: 535G2ABX9M) (FUCUS VESICULOSUS - UNII:535G2ABX9M) FUCUS VESICULOSUS 6 [hp_X] in 30 mL GALIUM APARINE WHOLE (UNII: Z4B6561488) (GALIUM APARINE WHOLE - UNII:Z4B6561488) GALIUM APARINE WHOLE 4 [hp_X] in 30 mL THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 6 [hp_X] in 30 mL MYOSOTIS ARVENSIS (UNII: C73BK97H5J) (MYOSOTIS ARVENSIS - UNII:C73BK97H5J) MYOSOTIS ARVENSIS 4 [hp_X] in 30 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 4 [hp_X] in 30 mL PODOPHYLLUM PELTATUM ROOT (UNII: 2S713A4VP3) (PODOPHYLLUM PELTATUM ROOT - UNII:2S713A4VP3) PODOPHYLLUM PELTATUM ROOT 6 [hp_X] in 30 mL CHONDRODENDRON TOMENTOSUM ROOT (UNII: 395A3P448Z) (CHONDRODENDRON TOMENTOSUM ROOT - UNII:395A3P448Z) CHONDRODENDRON TOMENTOSUM ROOT 6 [hp_X] in 30 mL APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 12 [hp_X] in 30 mL BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 3 [hp_X] in 30 mL EQUISETUM HYEMALE WHOLE (UNII: 59677RXH25) (EQUISETUM HYEMALE WHOLE - UNII:59677RXH25) EQUISETUM HYEMALE WHOLE 3 [hp_X] in 30 mL HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) (HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK - UNII:T7S323PKJS) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK 2 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-466-18 1 in 1 BOX 09/24/2020 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/24/2020 Labeler - Guna spa (430538264) Registrant - GUNA Spa (338587646) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-466)