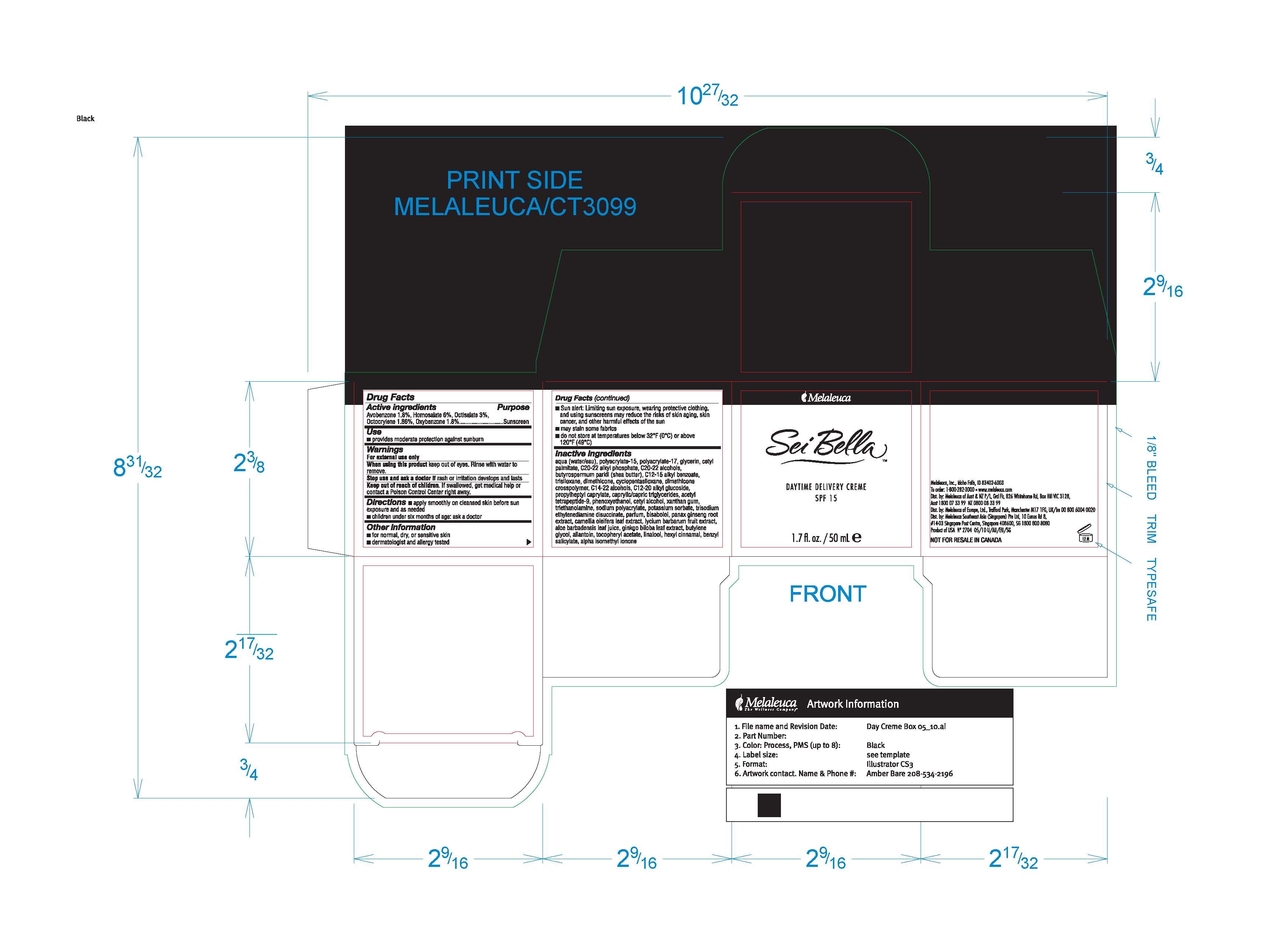
Label: SEI BELLA DAYTIME DELIVERY- avobenzone, homosalate, octisalate, octocrylene, oxybenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 54473-186-02 - Packager: Melaleuca, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 26, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- CLINICAL STUDIES
- GENERAL PRECAUTIONS
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients
aqua (water/eau), polyacrylate-15, polyacrylate-17, glycerin, cetyl palmitate, C20-22 alkyl phosphate, C20-22 alcohols, butyrospermum parkii (shea butter), C12-15 alkyl benzoate, trisiloxane, dimethicone, cyclopentasiloxane, dimethicone crosspolymer, C14-22 alcohols, C12-20 alkyl glucoside, propylheptyl caprylate, caprylic/capric triglycerides, acetyl tetrapeptide-9, phenoxyethanol, cetyl alcohol, xanthan gum, triethanolamine, sodium polyacrylate, potassium sorbate, trisodium ethylenediamine disuccinate, parfum, bisabolol, panax ginseng root extract, camellia oleifera leaf extract, lycium barbarum fruit extract, aloe barbadensis leaf juice, ginkgo biloba leaf extract, butylene glycol, allantoin, tocopheryl acetate, linalool, hexyl cinnamal, benzyl salicylate, alpha isomethyl ionone
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SEI BELLA DAYTIME DELIVERY
avobenzone, homosalate, octisalate, octocrylene, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54473-186 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.9 g in 50 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 3 g in 50 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 1.5 g in 50 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 0.93 g in 50 mL Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.9 g in 50 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) CETYL PALMITATE (UNII: 5ZA2S6B08X) SHEA BUTTER (UNII: K49155WL9Y) C12-15 ALKYL BENZOATE (UNII: A9EJ3J61HQ) TRISILOXANE (UNII: 9G1ZW13R0G) DIMETHICONE (UNII: 92RU3N3Y1O) Cyclomethicone 5 (UNII: 0THT5PCI0R) C14-22 ALCOHOLS (UNII: B1K89384RJ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) Cetyl Alcohol (UNII: 936JST6JCN) XANTHAN GUM (UNII: TTV12P4NEE) Trolamine (UNII: 9O3K93S3TK) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) LEVOMENOL (UNII: 24WE03BX2T) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) ALOE VERA LEAF (UNII: ZY81Z83H0X) GINKGO (UNII: 19FUJ2C58T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALLANTOIN (UNII: 344S277G0Z) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ASIAN GINSENG (UNII: CUQ3A77YXI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54473-186-02 1 in 1 BOX 1 50 mL in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/01/2010 Labeler - Melaleuca, Inc. (139760102) Establishment Name Address ID/FEI Business Operations Melaleuca, Inc. 139760102 manufacture