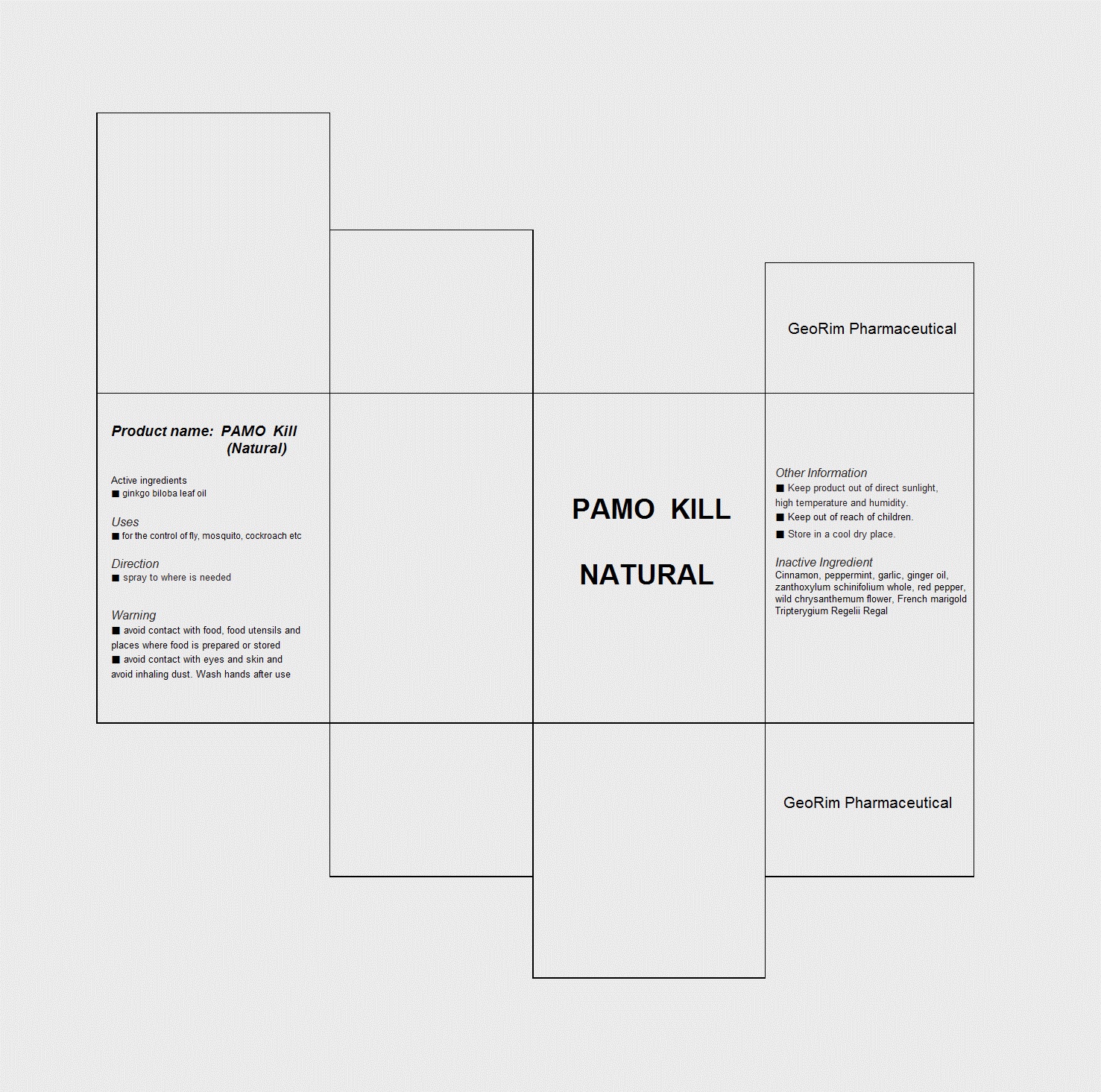
Label: PAMO KILL NATURAL- ginkgo biloba leaf oil liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 59494-1001-1 - Packager: GeoRim Pharmaceutical
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 2, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PAMO KILL NATURAL
ginkgo biloba leaf oil liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59494-1001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GINKGO BILOBA LEAF OIL (UNII: Y5967KO1JH) (GINKGO BILOBA LEAF OIL - UNII:Y5967KO1JH) GINKGO BILOBA LEAF OIL 1 g in 100 mL Inactive Ingredients Ingredient Name Strength CINNAMON (UNII: 5S29HWU6QB) PEPPERMINT (UNII: V95R5KMY2B) GARLIC OIL (UNII: 4WG8U28833) GINGER OIL (UNII: SAS9Z1SVUK) ZANTHOXYLUM SCHINIFOLIUM WHOLE (UNII: M8QGZ6O788) RED PEPPER (UNII: 6M47G7C4SY) DENDRANTHEMA INDICUM FLOWER (UNII: I6OER6U04L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59494-1001-1 100 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/08/2013 Labeler - GeoRim Pharmaceutical (688733165) Registrant - GeoRim Pharmaceutical (688733165) Establishment Name Address ID/FEI Business Operations GeoRim Pharmaceutical 688733165 manufacture(59494-1001)